



Ramesh, S.R., and W.-E. Kalisch. 1999. SDS-PAGE technique for demonstrating sex linked genes.
Dros. Inf. Serv. 82: 134-136. View PDF
Classical experiments to demonstrate the inheritance of X-chromosomal genes involve reciprocal crosses to compare heterozygous F1-female and hemizygous F1-male flies phenotypically, the latter ones getting their sex linked genes exclusively from their mothers. Here, we describe a simple technique involving the use of a 13.7% SDS-PAGE (Sodium Dodecyl Sulfate – PolyAcrylamide Gel Electrophoresis) to demonstrate electrophoretically the sex linkage of several genes coding for major glue protein fractions in the larvae of D. n. nasuta and D. n. albomicans. Interspecific crosses of these two Drosophila species belonging to the nasuta subgroup of the immigrans group are used for the following reasons: (1) The nasuta subgroup members show species-specific glue protein patterns. The major glue protein fractions are very prominent; therefore, one pair of salivary glands (from a single individual) is sufficient for obtaining the electrophoretic pattern (Ramesh and Kalisch, 1987). (2) The major glue protein fractions in D. n. nasuta and D. n. albomicans show different kd-values, as a result of which the patterns of protein fractions can easily be analyzed in the heterozygous F1-females (Ramesh and Kalisch, 1988).
We crossed the species D. nasuta albomicans (stock number: 15112-1751.0, obtained from the National Drosophila Species Resource Center, Bowling Green, Ohio, USA) and the D. nasuta nasuta [stock Mysore I from our lab (Ramesh and Kalisch, 1988), but the 15112-1781.0 stock from Bowling Green can also by used]. Standard cornmeal medium was used; cultures and crosses were maintained at 22±1oC.
The advantages of this experiment for teaching purposes are the little amount of tissue required (i.e., only one larva is needed) and the clear identification of the prominent glue protein fractions, by which, an unequivocal interpretation of the results is possible even by beginners.
Preparation of samples: Samples from third-instar larval salivary glands from parental and F1-hybrid cultures are prepared. Well-grown larvae are washed, separated according to the sex, and dissected in a 0.03 M phosphate buffer (pH 6.8) with 0.04 M KCl, 0.011 M NaCl, 0.003 M CaCl2, and 0.021 M MgCl2 (Ashburner, 1970), at 20±1oC. The glands are transferred for 20 min. into a 1.5 ml microfuge tube filled with cold 10% TCA. The tissue is washed with the help of a Pasteur pipette (each time for 20 min.) in 95% ethanol, a mixture of methanol and chloroform (1:1); finally it is dried at 37oC (which requires about 15 min.). To the dried tissue, 30ml of sample buffer [0.0625 M Tris-HCl (pH 6.8), 1% SDS, 1% b-mercaptoethanol, 10% glycerol, and 0.001% bromophenol blue] is added and the lid of the microfuge tube is tightly closed. After 2-3 hr., it is heated in boiling water for 10 min., cooled to room temperature and then centrifuged for 3 min. at 1,000 rpm. These extracts can be stored at -20oC for 8-10 days.
| Solutions | Lower gel | Upper gel |
|---|---|---|
| Lower gel buffer | 7.5 ml | — |
| Upper gel buffer | — | 4.0 ml |
| Acrylamide solution | 18.0 ml | 4.0 mlM |
| Dist. Water | 4.5 ml | 7.76 ml |
| 1% Ammonium persulfate | 200.0ml | 160 ml |
| 20% SDS | 225.0 ml | 240.0 ml |
| TEMED | 20.0 ml | 16.0 ml |
Solutions required for preparation of the gels: (1) lower gel buffer: 18.15 g Tris dissolved and made up to 100 ml with dist. water, pH adjusted to 8.8 with conc. HCl; (2) Upper gel buffer: 6.0 g Tris dissolved and made up to 100 ml with dist. water, pH adjusted to 6.8 with conc. HCl; (3) Acrylamide solution: 22 g acrylamide and 0.8 g bis acrylamide dissolved and made up to 100 ml with dist. water; (4) Ammonium persulfate: 1% solution (should be prepared fresh); (5) Sodium dodecyl sulfate: 20% solution.
Stacking gel and separating gels are prepared by mixing different proportions of solutions as shown in Table 1. The amount of gel solution mixtures are enough for casting 15 x 10 cm gels of 1 mm thickness. For details with regard to gel casting, see Ostermann (1984). It is preferable to cast the gels in cold. The prepared gel may be used immediatelyor may be stored at 8-10oC for 24-48 hr.
Electrophoresis: 15ml of the samples prepared is injected into each slot of the gel, which are then carefully layered with running (tray) buffer [0.05 M Tris, 0.384 M glycine buffer (pH 8.3) containing 0.1% SDS]. The electrophoresis is performed with 165 volts (35 mA max.) for 3 ½ hr at 20±1oC. The gel is treated overnight with a prestaining solution [50% TCA and isopropanol (1:1)]. 0.175% Coomassie brilliant blue R-250 (in 50% methanol and 10% acetic acid) is used for staining the gel for 2 hr., and a different mixture (25% methanol and 7.5% acetic acid) is used for destaining.
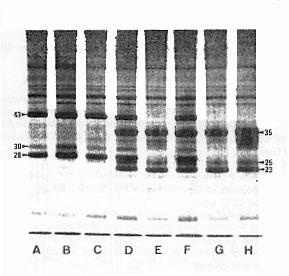 Figure 1. X-chromosomal linkage of major glue protein fractions in D. n. nasuta and D. n. albomicans. SDS-PAGE patterns of protein extracts from salivary glands of third-instar larvae. (A) male and (B) female of D. n. nasuta. (C) F1-male and (D) F1-female from a D. n. nasuta mother and a D. n. albomicans father. (E) F1-male and (F) F1-female from the reciprocal cross. (G) male and (H) female of D. n. albomicans. Note that in (C) as well as in (E) the F1-male shows the pattern of its mother, whereas in (D) as well as in (F) the F1-female shows a heterozygous pattern of both parents. Each protein extract is from one pair of salivary glands by the use of an individual larva. Differences in protein staining are based on gland size as well as species- and stage-specific development. Arrow-labeled bands indicate major glue protein fractions in both species which are coded by X-chromosomal genes. The approximate kd-values are given according to Ramesh and Kalisch (1988). Coomassie-blue staining. | Figure 1 shows the SDS-PAGE patterns of protein fractions from the salivary glands. The prominent bands between 43-23 kd belong among others to the glue proteins (Ramesh and Kalisch, 1987). It is evident from Figure 1, that the three labeled glue protein fractions in both species are produced by genes located in the X-chromosome, since the F1-hybrid males (C and E) show the phenotype of the P-females used for the cross; while the F1-females (D and F), irrespective of the direction of the cross, are heterozygous for the maternal and paternal patterns. Chromosomal linkage of the remaining major and minor glue protein fractions will be published elsewhere (Ramesh and Kalisch, in prep.). Acknowledgement: S.R.R. is grateful to the Univ. Grants Commission, New Delhi, India, for sponsoring and to the Deutscher Akademischer Austauschdienst, FR Germany, for the award of a scholarship. This study is part of a project (Ka 309/9-1) supported by the Deutsche Forschungsgemeinschaft. References: Ashburner, M., 1970, Chromosoma 31: 356-376; Osterman, L.A., 1984, In: Methods of Protein and Nucleic Acid Research, Springer-Verlag, Berlin and N.Y., p21-25; Ramesh, S.R., and W.-E. Kalisch 1987, Dros. Inf. Serv. 66: 117; Ramesh, S.R., and W.-E. Kalisch 1989, Genetica (in press). |