



Sperlich, D. 1999. Useful population cage experiments for demonstrating directional and balancing selection.
Dros. Inf. Serv. 82: 136-137. View PDF
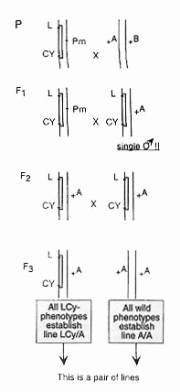 Figure 1. Crossing procedure for the construction of “monchromosomal” and “polychromosomal” populations. |
In a basic course in population genetics we are using successfully for years a simple cage experiment to demonstrate the effect of natural selection in populations carrying a recessive lethal. Depending on the population system the lethal is either going to be balanced or eliminated (Sperlich and Karlich, 1970). Strains used for the experiment are: a wild-type strain of D. melanogaster (D. pseudoobscura can be also used; see Sved and Ayala, 1970) and a L Cy/Pm strain with good expression of the markers (occasional selection of the strain for good manifestation of Cy is recommended). Using the ordinary marker strain technique pairs of lines are established (see Figure 1) carrying the same wild chromosome II in homozygous condition (+A/+A) or in combination with L Cy (L Cy/+A). At least ten such pairs of lines (A/A – L Cy/A, B/B – L Cy/B …, J/J – L Cy/J) must be available for the experiment. Lines with homozygotes A/A being lethal (about 30 percent!) must be discarded. “Monochromosomal” populations are then started each by founder flies from one pair of the lines only; e.g., line C/C and line L Cy/C. Wild flies are taken from the C/C line and L Cy-phenotypes from the L Cy/C line (there are wild-type flies in this line too which must be either discarded or counted as “wild”-type C/C). The ratio between C/C and L Cy /C genotypes in the founder population is chosen 2:1. “Polychromosomal” populations are founded in the same way but wild-type flies are now taken equally from all different pairs of lines (A/A, B/B, … , J/J) and L Cy flies from the corresponding L Cy-line (L Cy/A, L Cy/B, … , L Cy/J). The ratio wild: L Cy is again 2:1. Any population cage system can be used to keep the populations for three to four months. The temperature should be 25oC which ensures good manifestation of Cy and a generation time of about 15 days. Egg samples should be taken every second generation (this means monthly) by inserting 4-6 fresh vials into the cage for 24 hours. The flies hatching from these samples are then counted. The relative frequency of the L Cy chromosomes in the cage populations is easily calculated. Three to four samples are usually enough to demonstrate that L Cy is almost completely eliminated in this short period from the gene pool of the polychromosomal populations but appears balanced in the monochromosomal populations (Sperlich and Karlich, 1970; Sved, 1971). Starting with a L Cy frequency of q = 0.167 (= 1/6) it becomes nearly zero in the polychromosomal and around q = 0.25 to almost q = 0.50 in the monochromosomal populations. |
| A/A | LCy / A | LCy / LCy | ||
|---|---|---|---|---|
| Fitness | W | 1 | 0 | Population fitness = |
| Frequency | p2 | 2pq | q2 | =W · p2 + 2pq |
| (q0 = .1667; w = 1.4, 1.2, 1.0, 0.8, 0.6, 0.4, 0.2, 0.0) |
The population system is very simple since L Cy/ L Cy phenotypes are completely lethal. Putting the relative fitness of the heterozygotes (e.g., L Cy/A) first, only the fitness of the wild-type “homozygotes” (e.g., A/A in mono. and A/B … D/D in poly.) remains unknown. Starting with q = 0.167, the following fitness distribution calculations can be easily made with any pocket calculator by the students: By iteration of this formula and by using different W values students can easily gain basic understanding for selection processes maximizing population fitness. Additional discussions arise automatically about genetic load, balancing selection and computer simulations of population systems (Sperlich et al., 1982).
References: Sperlich, D., and A. Karlik 1970, Genetica 41:265-304; Sved, J., 1971, Genet. Res. 18: 97-105; Sved, J., and F.J. Ayala 1970, Genetics 66: 97-113; Sperlich, D., A. Karlik, and P. Pfriem 1981, BiolZbl. 101: 395-411.