



Lilly, Brenda, and Juan Botas. 1999. An efficient mutagenesis screen to generate duplications of polytene section 8 on the X chromosome of Drosophila melanogaster.
Dros. Inf. Serv. 82: 105-112. View PDF
Duplications of the X chromosome are invaluable tools for conducting
detailed genetic analysis of X chromosome genes.
Appropriate duplications are essential for defining complementation
groups within a given interval. Although
77% of the euchromatic X chromosome is covered by duplications, several regions
remain devoid of these tools. Interval 8B is one such region, which prompted us to design
a method to create a duplication that was specific to this segment. We initiated a genetic screen to isolate
duplications that covered the 8B region.
Our strategy utilized an X-Y translocation and an attached X-Y chromosome
to generate viable pieces of the X chromosome, connected to a complete Y chromosome.
Our results demonstrate an efficient method for generating duplications
of the X chromosome and describe five new duplications that span region 8B. Two of these duplications bridge the gap
between previously described duplications, while the other three cover only
a portion of these segments. These new duplications provide important tools
that will permit a systematic genetic analysis of the 8B interval to be conducted.
1. INTRODUCTION
Screening for lethal mutations on the X chromosome requires that the region of interest be covered by a duplication that resides elsewhere within the genome. These duplicated segments enable lethal mutations to be carried in hemizygous males that possess only one X chromosome. By carrying mutations in males, complementation tests can then be performed to facilitate the characterization of alleles within a given interval. Unfortunately, regions that lack appropriate duplications cannot be genetically dissected, because complementation analysis is not possible. Thus, the availability of appropriate duplicated segments is essential for conducting detailed genetic analysis of X chromosome genes. Studies have shown that approximately 77% of the euchromatic X chromosome is covered by duplications (Hilliker et al., 1980; Eberl et al., 1992).
One region which is devoid of available duplications is the 8B interval. Our interest in this region initiated with the observation that a cDNA sequence that we had identified, termed dlim1, mapped to this segment. The results of which will be presented elsewhere (Lilly et al., 1999, submitted). To our knowledge all pre-existing duplications that spanned this segment had been lost and were unavailable for our use. As a consequence, to carry out a genetic analysis of the region we were compelled to generate a duplication that covered the 8B interval. We designed a screen to create a X chromosome duplication on a complete Y. Y-linked X chromosome duplications are particularly useful because they segregate from the X chromosome in males and facilitate complementation analysis.
The strategy that we used was based on the method of Brosseau et al., (1960), in which they described the generation of Y chromosomes bearing specific sections of the X chromosome. The design of their screen was dependent upon the fulfillment of two requirements. First, that the translocations used had breakpoints near the region of interest. Secondly, that the irradiated translocations retained small enough fragments to prevent hyperploidy. Using this as a framework, our screen took advantage of a pre-existing X-Y translocation with breakpoints near 8B. By inducing a recombination event between our translocation and an attached X-Y chromosome, we provided an intact Y chromosome. The complete Y chromosome supplied the neccessary elements for proper segregation and fertility. Using the recombined version of the translocation chromosome, we then induced breaks by g rays and screened for viable males. Viable males with potential 8B duplications were first screened for their ability to complement oc[1] in 8A, and subsequently with neighboring alleles.
From our screen we generated five duplications that complemented oc[1] and extended into region 8B. Our results describe an efficient method for generating viable pieces of the X chromosome attached to a complete Y chromosome. The strategy we used allowed us to screen very few flies to recover several individual chromosomes with the region of interest. The duplications that were generated cover segments spanning 8B, a region which had previously lacked available duplications. These new duplications provide additional useful tools for the genetic analysis of genes that reside within this interval. These tools will allow for a systematic complementation analysis to be achieved, and facilitate the characterization of genes within region 8B
(i) Fly stocks
All fly strains were maintained on standard cornmeal,
molasses, yeast, and agar medium. Detailed description of most stocks can be found in Lindsley
and Zimm (1992) and/or in Flybase. Stocks
used in this study were, y[1] btd[1]/FM7c; y[1] lz[89d18-15] f[1]/Dp(1;Y)lz; ct[n]
oc[1]/FM1; and lz[K]; obtained
from Bloomington stock center. Stocks,
FM4, w[1] B[+]/T(1;Y)156, y[1] B[S]; 0/C(1)M4, y[2]/XYL-YS, y[2] su[wa] wa;
C(1)RM, y/XYL-YS, y[2] su[wa] wa;
0/C(1)RM, y[1] v[1] bb[**]/C(1;Y)129-16, y[2] y[+] su(w[a]) w[a]; and
w[1] otd[1]/FM7c were obtained from Mid-American
stock center. C(1)DX,
y[1] w[1] f[1]; Dp(1;2)FN107/bw[D] was
provided by Robert Finkelstein. The
Nrg allele, In(1)RA35/FM7c, was obtained from Corey Goodman. New chromosomes generated in this study
are diagrammed in Figures 1 and 3.
(ii) Mutagenesis screen
For the duplication screen, 2100 males of genotype, XYL-YS, y[+]
T(1;Y)156, y[1] B[S] carrying the newly
recombined translocation, diagrammed in Figure 1, were subjected to 3500 rad
of gamma irradiation (Gammacell-1000).
The irradiated males were allowed to recover for eight hours, and then
mated en masse to y[1]
w[1] females for 72 hours, after which the males were discarded.
Females were allowed to lay eggs for another 96 hours, 7 days in total. Individual F1 viable male progeny from this cross, that possessed
at least one of the Y chromosome markers, B[S] and y[+],
were crossed to three females of genotype, ct[n] oc[1]/FM1. F2 male
progeny from this cross carrying the ct[n] oc[1] chromosome and a putative duplication on the Y, were
screened for the presence of ocelli, indicating the duplication contained
X chromosome material that rescued the locus. The F2 males carrying duplications that rescued the oc phenotype were established as balanced stocks. Duplications were maintained using the
compound chromosome C(1)DX, y[1] w[1] f[1] in females, and an embryonic lethal allele, otd[1] in males.
(iii) Complementation tests
Complementation tests to map the breakpoints of the duplications were
conducted similar to those for the oc[1] allele
in Figure 2. Males carrying the
newly generated duplications Dp(1;Y)
and otd[1] were crossed to females
carrying the allele of interest, and an X chromosome balancer. The ability of the duplications to complement
lethal alleles was determined by the presence of male progeny
that carried markers for the duplication, y[+] B[S], and for the allele of interest. For the viable lz[K] allele, all males were screened for the rescue of the
rough eye lozenge phenotype. For each cross greater than 100 progeny were
scored.
| 3. RESULTS (i) Generation of a recombined X-Y translocation |
 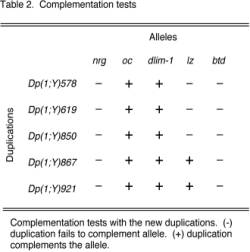 |
The starting translocation is shown in Figure 1A. Since our interest was in the 8B region we sought to place the portion of the X chromosome 7D-20 containing 8B on to a complete Y chromosome. To do this we recombined the sequences of the translocation T(1;Y)156 with that of an attached X-Y chromosome, XYL-YS 129-16, y[2] y[+] su(w[a]) w[a] (Figure 1B). By doing so, we generated a translocation that contains the original short arm of the Y chromosome attached to X chromosome 1-7C, and a newly recombined chromosome. The recombined chromosome contained the original long arm of the Y chromosome attached to X chromosome 7D-20, but was now joined to a complete Y chromosome from the attached X-Y chromosome (Figure 1E). The newly recombined translocation was designated, XYL-YS, y[+] T(1;Y)156, y[1] B[S]. Each recombined translocation was established as an individual stock using a compound chromosome C(1)M4, y[2]. Because the two pieces of the translocation segregated independently, the stock consisted of females with the compound chromosome C(1)M4 and Y[S] with X chromosome 1-7C. The males of this stock contained both portions of the X-Y translocation (Figure 1E). All other combinations of these three chromosomes resulted in non-viable progeny which permitted the stock to be balanced. The females of this stock, containing the compound chromosome and a part of the translocation required additional care to maintain. Presumably the combination of the translocation segment 1-7C, and the compound chromosome had an adverse affect on their viability. With the generation of the recombined translocation we had the primary tool that we needed for the production of duplications that covered our region of interest, 8B.
(ii) Mutagenesis screen for X chromosome duplications
Our mutagenesis screen was designed to induce double
strand breaks in the newly recombined chromosome, that would generate viable
pieces of the translocation in males that possessed an additional X chromosome.
By using a translocation that had breakpoints near region 8B, we essentially
predetermined the distal breakpoint.
Therefore, our screen was simplified by having only to generate a proximal
breakpoint that was small enough to be compatible with viability. Since the two pieces of the translocation segregated independently
from one another, it allowed us to screen by markers for viable males that
carried the portion of the translocation that contained region 8B.
Males that retained too much of the translocation would not survive
as a consequence of hyperploidy (Patterson et al., 1937).
Thus, only males that had lost a considerable part of the X chromosome
material would be viable. This
provided us with a powerful and efficient screen by eliminating all males
that retained unuseable translocations.
The design of the screen is diagrammed in Figure 2. Males carrying the translocation were subjected to g irradiation, allowed to recover and crossed to y[1] w[1] females. By discarding the irradiated males after 72 hours we insured that the resulting progeny would be derived from irradiated sperm. As expected, the majority of the progeny recovered were males. Table 1 summarizes the results of the mutagenesis screen. 2100 irradiated males produced 1608 viable males, possessing four different phenotypes. Only 15 females were recovered, and were probably a result of low frequency non-disjunction from the parents. The four phenotypic groups of the males recovered (Table 1), reflected the probable outcomes resulting from chromosome breaks due to the g rays. As shown in Figure 2 the dominant markers, y[+] and B[S] were transmitted from the Y portion of the recombinant translocation. Males that lacked both of these markers made up 69% of the viable progeny. The absense of these markers in viable males suggested that these flies had recieved translocations that had lost significant portions of the chromosome, or lacked it altogether. The three other phenotypes, making up 31% of the progeny, contained one or both of the dominant markers, y[+] and B[S]. However as a consequence of hyperploidy, these males must have lost most the X chromosome to be viable. Surprisingly, no males were recovered that retained the w[+] marker of the smaller translocation. The reason for this is unclear; however, its proximity to the tip of the X may have reduced the number events which would allow for its transmission.
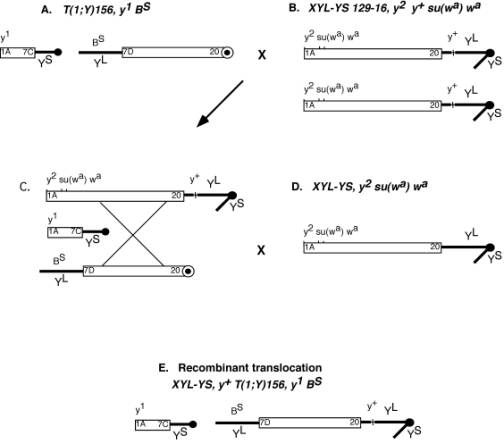 |
Figure 1. Generation of a recombined translocation chromosome. Open boxes represent X chromosome material and thick black lines represent Y chromosomes. (A) Males carrying translocation T(1;Y)156, y[1] B[S] were crossed to females (B), XYL-YS 129-16, y[2] y[+] su(w[a]) w[a], having two attached X-Y chromosomes, to generate females, with an attached X-Y and the translocation (C). These females, from which the recombination event was designed to occur, were crossed to males (D), XYL-YS, y[2] su(w[a]) w[a], an attached X-Y chromosome. Recombination events were identified by the presence of y[+] and B[S] markers segregating together in males (E). |
The males that displayed one or more of the translocation markers with which the 8B region segregated with, y[+] and B[S], shown in Figure 2C were crossed to oc[1] females. 495 males were crossed to oc[1] females, of which only 153 were fertile and produced progeny. From the 153 crosses, five individual lines rescued the oc[1] phenotype and were presumed to contain a duplication of the 8A1 region of the X chromosome. Four of these retained the y[+] and B[S] markers, and were designated, Dp(1;Y)578, Dp(1;Y)619, Dp(1;Y)867, and Dp(1;Y)921, while Dp(1;Y)850 retained only the y[+] marker and lacked B[S]. Males with duplications that rescued oc[1] were maintained as stocks over a lethal oc allele, otd[1] in males and a compound X chromosome, C(1)DX in females. To verify the regions that these duplications covered, the five duplications were mapped by complementation tests using surrounding alleles outlined in Table 2.
(iii) Complementation tests of duplications
In generating duplicated segments that complemented
the oc[1] allele, we had predicted that the neighboring region,
8B would be included in one or more of these new duplications. To determine if the 8B region was covered,
and to define the breakpoints of these duplications, we carried out complementation
tests using alleles that surrounded the oc locus at region 8A. Because of the lack of duplications that span 8B-C, few alleles
exist that have been cytologically mapped to this region. For purposes of clarity we used only well
characterized alleles whose cytological locations had been verified by molecular
means for our complementation analysis. Table 2 outlines the results of the complementation tests. The most distal and most proximal alleles
that failed to complement these duplications are shown from left to right,
respectively. Figure 3 illustrates
the mapping data of the five new duplications. Shown are two previously described duplications
that flank the 8B region. Dp(1;2)FN107,
covers the X chromosome region 7A8-8A5 (Craymer and Roy, 1980), and Dp(1;Y)lz spans region 8D(7-9)-9A(4-5) (Santamaria and Randsholt,
1995).
The new duplications span the gap that was previously not covered by any available duplications. All five duplications have a distal breakpoint that lies between Nrg at 7F1, and oc located at 8A1. This is not surprising, considering the original translocation had a breakpoint that was reported to be near 7D (Lindsley and Zimm, 1992). The duplications overlap Dp(1;2)FN107 and fill the gap that had existed within this interval. The proximal breakpoints of these five duplications vary to a much greater degree. Two duplications Dp(1;Y)867 and Dp(1;Y)921 complement lz, and thus overlap with the previously described duplication Dp(1;Y)lz. Neither of these two duplications complement the btd allele, indicating that their breakpoints lie between the interval 8D(8-9) and 9A1. The three other duplications, Dp(1;Y)578, Dp(1;Y)619, and Dp(1;Y)850, failed to complement the lz allele. The most proximal allele tested, for which these duplications complement is a newly described gene, dlim1, (Lilly et al., 1999, submitted) which has been mapped to region 8B(1-2) by cytology and deficiency analysis. Thus, the proximal breakpoint of these duplications lies somewhere between 8B(1-2) and the lz allele at 8D(8-9). Using a host of other alleles scattered along the X chromosome, these duplications failed to complement any of them. This suggests that the duplications are made up of a contiguous stretch of the X chromosome within the 8A-B region and lack any other X chromosome material. In addition, each new duplication was analyzed by cytology using the dlim1 cDNA, as a marker. Because the Y chromosome does not polytenize it was difficult to map the duplicated segments by cytology. The analysis of the squashes supported our complementation data in that we were able to see pieces of the X chromosome that hybridized with our probe (not shown).
4. DISCUSSION
To facilitate the analysis of a newly discovered gene found in the
8B region of the X chromosome, we undertook a genetic screen to create duplications
that covered interval 8B. This
region has lacked sufficient characterization because of the absence of duplications
that span it. Duplications of
the X chromosome enable one to test by complementation the allelelism of mutations
within a given region. Thus,
without appropriate duplicated segments, comprehensive genetic screens can
not be performed. To generate
new duplications we utilized an efficient mutagenesis scheme, that was based
on that described by Brousseau et al.,
(1961). The screen design took
advantage of a pre-existing X-Y translocations with breakpoints near region
8B, and the lethality associated with hyperploidy of the Drosophila X chromosome.
Through a recombination event we attached an intact Y chromosome to
the translocation which was a neccessary element for generating usuable duplications.
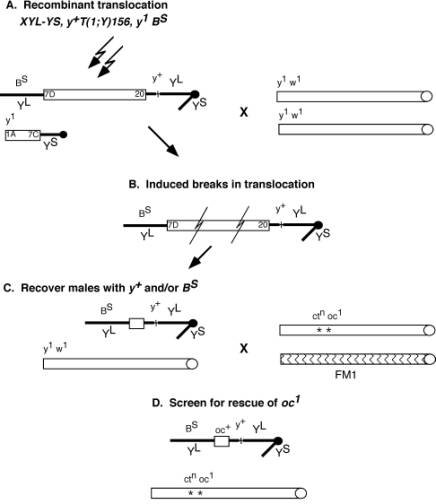 |
Figure 2. Mutagenesis screen for X chromosome duplications. Open boxes represent X chromosomes and thick black lines represent Y chromosomes. (A) Males carrying the recombined translocation chromosome were subjected to g irradiation and crossed to y[1] w[1] females. (B) Chromosomal breaks produced viable male progeny that retained one or both markers from the recombined translocation chromosome. These males (C), shown with both the y[+] and B[S] markers, were crossed to ct[n] oc[1]/FM1 females. The progeny from this cross were screened for rescue of the oc phenotype (D). The diagrammed duplicated segment (C), represents just one example of the potential viable chromosomes. Many other possibilities exist due to the random breaks induced by the g rays. Only males with a y[+] and/or B[S] markers were crossed to ct[n] oc[1] females. |
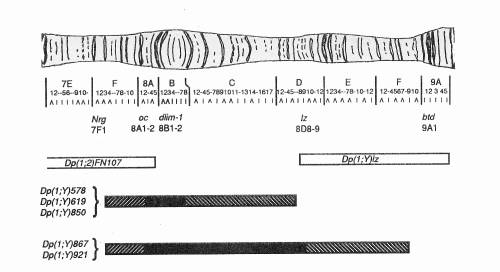 |
Figure 3. Cytogenetic map of the interval covered by the duplications. The breakpoints of the five new duplications were defined by complementation tests with alleles surrounding oc. The alleles used are shown with their map position along the chromosome. Two preexisting duplications are shown as open boxes. The distal duplication, Dp(1;2)FN107 has breakpoints, 7A8-8A5, and covers oc. The proximal duplication, Dp(1;Y)lz has breakpoints, 8D(7-9)-9A(8-9), and covers lz and btd. The new duplications are represented as filled boxes. All have a distal breakpoint between Nrg, 7F1 and oc, 8A1. Three of these duplications have proximal breakpoints between dlim1, 8B(1-2) and lz, 8D(8-9). The two remaining duplications break proximally between lz and btd (9A1). The strips within the boxes represent the region of uncertainty in the breakpoints. |
From this screen we generated five new Y-linked duplications that covered the 8B interval. Of these duplications, two span a gap of the 8B region that covers an uncharacterized region. The other three duplications break within this interval and cover smaller portions of the 8A-C region. Our method allowed us to generate duplications of the 8B region with great efficiency and specificity. By taking advantage of the lethality caused by hyperploidy and the translocation breakpoints near 8B, our screening process was highly selective. This scheme could be modified and used for other selected regions of the X chromosome. The duplications that were generated and described here provide us with valuable tools for dissecting out the genetics of the X chromosome. This study should greatly facilitate further genetic and molecular analysis of loci mapping within this region.
Acknowledgments: The authors thank the Bloomington and Mid-America stock centers for providing fly stocks. We wish to acknowledge Robert Finkelstein and Corey Goodman for providing alleles used in this study. BL was supported by a National Institutes of Health postdoctoral fellowship. This work was supported by grants from the National Institutes of Health and the National Science Foundation to JB.
References: Brosseau, G.E., B. Nicoletti, E.H. Grell, and D.L. Lindsley 1961, Genetics 46: 339-346; Craymer, L., and E. Roy 1980, Dros. Inf. Serv. 55: 200-204; Eberl, D.F., A. Perkins, M. Engelstein, A.J. Hilliker, and N. Perrimon 1992, Genetics 130: 569-583; Hilliker, A.J., R. Appels, and A. Schalet 1980, Cell 21: 607-619; Lindsley, D.L., and G.G. Zimm 1992, The Genome of Drosophila melanogaster. Academic Press, New York; Patterson, J.T., W. Stone, and S. Bedicheck 1937, Genetics 22: 407-426; Santamaria, P., and N.B. Randsholt 1995, Molec. Gen. Genet. 246: 282-290.