Genetic stability
under stresses expected in a space station environment: Effect of hypergravity and vibration in
Drosophila melanogaster.
Thompson,
James N., jr. 1,
R.C. Woodruff 2,
Jenna J. Hellack 3,
Tera L. Beaird 1,
Gerald P. Camren 1,
Wade P. Dressler 1,
Ann Gettys1, Greg S. Hendrix 1,
D. Jeremy Madrid 1,
Matthew J. Potthoff 1,
H. Nathanial Scott 1,
Morsal R. Tahouni1, and Brian T. Torgerson 1. 1
Department of Zoology, University of Oklahoma, Norman, OK 73019;
2 Department of Biological
Sciences, Bowling Green State University, Bowling Green, OH
43403; 3
Department of Biology, University of Central Oklahoma, Edmond, OK 73034.
Genetic and developmental systems will be challenged by new stresses
when organisms begin to adapt to long-term habitation of a space environment,
such as that on the International Space Station (ISS).
Previous assays of mutation and chromosome damage in response to hypergravity
or stress (Pence, 1999) have yielded conflicting results or have used extremes
that are unlikely to be experienced in actual space exposures.
Our initial ground-based studies in Drosophila melanogaster are designed to estimate
mutation rates, aneuploidy, somatic mutation, and developmental stability
under some of the stress exposures that organisms can experience in a space
environment like that on the ISS. In
addition to providing valuable information about genetic and developmental
stability and about the capacity of an organism to adapt to a space environment,
these experiments yield initial ground control data for possible multi-generation
mutation rate experiments on the ISS or other space environment.
Experimental cultures were exposed to hypergravity and vibration stresses
at NASA/Ames Research Center, and genetic breeding programs were then completed
at the University of Oklahoma and at Bowling Green State University. The hypergravity conditions we have chosen
to test first are near the high end of the range that organisms might experience
on vehicle launch and travel. Drosophila
are exposed to hypergravity using the 1-Foot Diameter Centrifuge (2 - 5 g),
which is designed to maintain carefully regulated low-level hypergravity conditions
for extended periods. Our treatments
were typically 2-hour or 4-hour exposures, although pilot studies with other
treatments not reported here have also been done. Vibration conditions are
modeled on the mid-deck vibration of a shuttle launch (Figure 1). Vibration exposures (typically 5 minutes
in duration) were done on a computer-controlled Vibration Table (20 - 2000
Hz). “Full Range”
refers to a five-minute exposure to random vibration that has the cumulative
frequency profile shown in Figure 1.
Five-minute exposures to just low range (20-150 Hz), mid-range (150-1000
Hz), and high range (1000-2000 Hz) are also reported here.
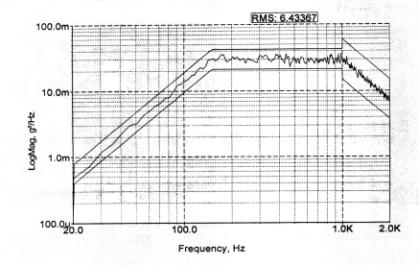
Figure 1. Random vibration profile (Full Range)
as established in Interface Definitions Document NSTS-21000-IDD-MDK for a
shuttle mid-deck. This representative
profile printout is from an experimental treatment conducted in January 2001
(Log #2529).
|
Table 1. Nondisjunction in the zeste test: Vibration and hypergravity.
|
Treatment
|
Normal Adults
|
Exceptional
|
Total
|
% Exceptional
|
|
Female
|
Male
|
|
|
|
|
|
|
|
|
Control
|
99,788
|
7
|
12
|
99,807
|
0.0190
|
|
|
|
|
|
|
|
|
Hypergravity:
|
|
|
|
|
|
|
2
g – 2 hr
|
17,089
|
0
|
3
|
17,092
|
0.0180
|
|
|
|
|
|
|
|
|
4
g – 2 hr
|
5,053
|
0
|
2
|
5,055
|
0.0400
|
|
|
|
|
|
|
|
|
5
g – 2 hr
|
24,762
|
5
|
9
|
24,776
|
0.0565a
|
|
|
|
|
|
|
|
|
5
g – 4 hr
|
114,627
|
12
|
31
|
114,670
|
0.0375b
|
|
|
|
|
|
|
|
|
Vibration:
|
|
|
|
|
|
|
Full
Range
|
79,143
|
5
|
22
|
79,170
|
0.0341c
|
|
|
|
|
|
|
|
|
20-150Hz
|
17,265
|
0
|
4
|
17,269
|
0.0232
|
|
|
|
|
|
|
|
|
150-1000
Hz
|
17,102
|
2
|
7
|
17,111
|
0.0526d
|
|
|
|
|
|
|
|
|
1000-2000
Hz
|
7,828
|
1
|
1
|
7,830
|
0.0260
|
|
|
|
|
|
|
|
|
Total:
|
|
32
|
91
|
382,780
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a Normal Test, P < 0.05; Fisher’s exact P = 0.003; c2 = 10.52, P < 0.005
b Normal Test, P < 0.05; Fisher’s exact P = 0.008; c2 = 6.29, P < 0.025
c Normal Test, P < 0.05; Fisher’s exact P = 0.034; c2 = 3.90, P < 0.05
d Normal Test, P < 0.05; Fisher’s exact P = 0.015; c2 = 6.87, P < 0.01
|
The
zeste
test (Zim-mering, et al., 1990) is a genetic breed-ing program designed to
detect chromo-some loss and gain in male and female progeny from parental
fe-males exposed to stress or control condi-tions. Data from the zeste test (Table 1) show an in-creased rate of aneuploidy from nondisjunction
in flies exposed to extended periods of 5 g (2h: 0.056%, P < 0.01; 4h:
0.038%, P < 0.01) and to vibration, particularly in the 150-1000 Hz range
(full range: 0.034%, P < 0.05; 150-1k range: 0.053%, P < 0.05), compared
to the control frequency.
Sex-linked
lethal mutation rate estimates using the Basc balancer stock indicate about a two-fold increase at 5 g (2h: 0.28%, P < 0.05; but marginally non-significant at 4h:
0.24%) compared to 1 g controls (0.14%).
The 2g, 4g, and vibration treatments may be slightly elevated, but
are not significantly different form the controls at the present sample sizes. Additional replicates will be completed
soon.
There
is no experimental evidence for chromosome breakage due to either hypergravity
or vibration stresses (Table 3), although there is significant chromosome
breakage caused by gamma radiation, as expected.
In conclusion, it appears that exposures to some of the stress conditions
that can be experienced in a space environment might cause an increase in
genetic damage, but the degree of that damage is not necessarily very large. This might help account for some of the
disagreement in results from earlier studies. Additional ex-periments are now being done to explore other
treat-ment levels and stresses, such as continuous expo-sure to low level
radiation, and possible interac-tion effects among these stress condi-tions.
|
Table 2. X-Linked lethals: Vibration
and hypergravity.
|
Treatment
|
Normal
|
New Lethals
|
Total
|
%
|
|
|
|
|
|
|
|
Control
|
10,859
|
15
|
10,874
|
0.1379
|
|
|
|
|
|
|
|
Hypergravity:
|
|
|
|
|
|
2
g – 2 hr
|
2,848
|
5
|
2,853
|
0.1752
|
|
|
|
|
|
|
|
4
g – 2 hr
|
1,347
|
2
|
1,349
|
0.1482
|
|
|
|
|
|
|
|
5
g – 2 hr
|
6,105
|
17
|
6,122
|
0.2777a
|
|
|
|
|
|
|
|
5
g – 4 hr
|
10,307
|
25
|
10,332
|
0.2420b
|
|
|
|
|
|
|
|
Vibration:
|
|
|
|
|
|
Full
Range
|
2,606
|
5
|
2,611
|
0.1915
|
|
|
|
|
|
|
a Normal Test, P < 0.05; Fisher’s exact P = 0.036; c2 = 4.07, P < 0.05.
b Normal Test, P < 0.05; Fisher’s exact P = 0.056; c2 = 3.04, P > 0.05.
|
Acknowledgments: We thank Tianna Shaw and Duncan
Atchison for facilitating our research at the NASA/Ames Research Center
(ARC), Sharmila Bhattacharya for valuable discussions and hospitality in
her genetics laboratory, Ruth Globus for access to the 1-Foot Centrifuge,
Chris Chen for assistance with the Vibration Table, and Max Sanchez for
technical assistance at the ARC. Wendal
Porter constructed the sample holder for the Vibration Table and has provided
other technical advice. Joe
Fleming provided laboratory support at the University of Oklahoma. This research is supported by NASA grant
NAG 2-1427.
References: Pence, M., 1999. Utilization of insect models in space
biology research applications. NASA
White Paper; Zimmering, S.,
C. Osgood, and J.M. Mason 1990. Mut. Res.
234: 319-326.
|
Table 3. Summary of spontaneous, gamma ray, hypergravity,
and vibration induced chromosomal breakage in Drosophila melanogaster
males using the hyperploidy test [C(1)DX, y w f females ´ treated Canton-S males] [scoring
female progeny].
|
|
# Breaks
|
# Chromosomes Scored
|
% Breakage
|
|
|
|
|
|
Spontaneous
|
|
|
|
|
Total from 8 replicates
|
0
|
10,184
|
0
|
Gamma Rays
|
|
|
|
|
1,000 R
|
2
|
275
|
0.73***
|
|
2,000 R
|
1
|
348
|
0.29*
|
|
4,000 R
|
|
|
|
|
Total from 2 replicates
|
11
|
1,962
|
0.56***
|
|
8,000 R
|
8
|
866
|
0.92***
|
Hypergravity
|
|
|
|
|
2g for 2 hours
|
|
|
|
|
Total from 2 replicates
|
0
|
7,230
|
0
|
|
5g for 2 hours
|
|
|
|
|
Total from 4 replicates
|
0
|
6,995
|
0
|
|
5g for 4 hours
|
|
|
|
|
Total from 1 replicate
|
0
|
458
|
0
|
|
8g for 1 hour
|
|
|
|
|
Total from 2 replicates
|
0
|
4,505
|
0
|
Vibration Full
|
|
|
|
|
Total from 2 replicates
|
0
|
7,257
|
0
|
|
|
|
|
|
*Fisher’s exact P < 0.05; ***P < 0.001.
|

