
Hodge, Simon. 2001. The effect of pH and water content of natural resources on the development of Drosophila melanogaster larvae. Dros. Inf. Serv. 84: 38-43.
|
|
|
|||
|
|
||||
Hodge, Simon. Department
of Entomology and Nematology, IACR-Rothamsted, Harpenden, Hertfordshire, AL5
2JQ, UK.
In nature, Drosophila utilize a variety of fermenting fruit, fungi, and other rotten vegetable matter as breeding sites. These resources vary in their physical structure and their chemical properties. Moisture content of the larval resource has been shown to affect development time, survival, pupal viability, pupal shape, and the body size of adult dipterans (Bay et al., 1969; Cook et al., 1980; David et al., 1983; Fatchurochim et al., 1989; Barnard and Harris, 1992). In Drosophila, water content of the resource affects larval feeding depth and pupation site (Sameoto and Miller, 1968; Arthur and Cassey, 1992) and the intensity and form of interspecific interactions (Arthur, 1996; Hodge and Mitchell, 1998). Hodge and Wilson (1997) found that larval survival in D. hydei and D. melanogaster larvae was reduced when the water content of laboratory medium was at extremes of high and low, though the response of D. melanogaster larvae was less pronounced than that of D. hydei.
In the laboratory, the pH of the culturing media has been shown to affect a variety of parameters in Drosophila life history, such as survival, development time, wing size, genetic stability, and pupation height (Goldat and Beliava, 1935; Gordon and Sang, 1941; Burdick and Bell, 1954; Hodge, 1995; Hodge et al., 1996; Hodge and Caslaw, 1998). Resource acidity has also been shown to affect the survival of larvae of other dipteran species (e.g., Morgan and Schmidt, 1966; Chan and Jang, 1995).
The aim of this investigation was to rear D. melanogaster larvae on
different fruits, and attempt to relate any differences found in life history
performance with the acidity and water content of these resources.
Ten types of fresh fruit were used in this experiment: orange, lemon, grapefruit, banana, cucumber, melon, tomato, avocado, pear, and apple. To provide a similarly homogeneous culture medium from each of the fruits, the fruits were peeled, chopped, and puréed using a pestle and mortar. Five grams of purée was placed into glass vials (70mm ´ 25mm diameter), with each fruit replicated six times. The pH of the fruit was measured using an electronic pH meter (Jenway 3015, Jenway Ltd., Essex, England) and 35 first instar D. melanogaster larvae were added to the vial. The larvae were less than 12 hours old at the time of introduction. The vials were stoppered with foam bungs and maintained in an incubator at 26+1oC, a relative humidity of 45+5%, and a light:dark cycle of 16:8 hours. The vials were checked daily and any emerged adults removed and stored in 75% methylated ethanol. The number of pupae in each vial was recorded and the percentage survivorship from larvae to pupae, pupae to adults, and larvae to adults was calculated. The mean development time to adulthood (MDT) of the larvae in each vial was calculated. Female wing length was used as a measurement of body size, with up to 10 females being measured from each vial. The measurement used was the length of vein 3 from the anterior crossvein to the wing tip. At the magnification used, 55 graticule units was equivalent to 1mm.
 The water content of each type of fruit was estimated by weighing,
drying at 45oC for at least 24 hours, and re-weighing until a constant
weight was achieved. Four replicates
of each fruit were used to calculate a mean water content.
The water content of each type of fruit was estimated by weighing,
drying at 45oC for at least 24 hours, and re-weighing until a constant
weight was achieved. Four replicates
of each fruit were used to calculate a mean water content.
The average values for the water content, pH, and D. melanogaster performance measures for each fruit are presented in Table 1. The water content ranged from 68% for avocado to 98% for cucumber, and the pH ranged between 2.4 for lemon and 6.8 for avocado. There were significant differences between fruits for all the D. melanogaster performance measures.
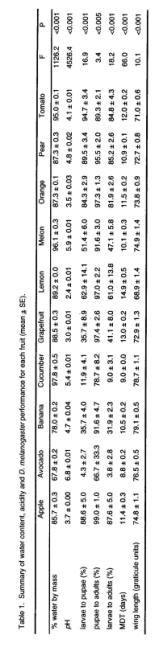
Survival of larvae to adulthood was highly correlated with the survival
of larvae to the pupal stage (r = 0.994, P < 0.001; see Figures 1a and
1b). Larval survival showed no significant
relationship with resource pH. The relationship between larval survival
to adulthood and resource water content was approximately parabolic, with
the highest survival occurring between 85% and 95% water content by mass (Figure 1b). Survival appeared to drop at both extremes
of water content and a weak, though significant, quadratic curve could be
fitted to the data (y = -0.23x2 + 40.3x – 1656.4; r = 0.74, P < 0.05).
The viability of pupae was lowest on the driest and wettest resources, and the relationship between pupal viability and the water content of the resource could be described well by a quadratic equation (y = -0.1x2 + 17.1x – 635.8; r = 0.97, P < 0.001; Figure 1c). There was also a significant negative correlation between pupal viability and resource pH (y = -1.94x2 + 11.8x + 79.3; r = 0.86, P < 0.01; Figure 2a).
The relationship between the MDT of the larvae and the water content of the resource again appeared parabolic, with the quickest development occurring in the driest and wettest resources (y = -0.013x2 + 2.25x - 83.53; r = 0.69, P < 0.05; Figure 1d). The MDT was negatively correlated with resource pH; the more acidic the media the longer the MDT (y = -1.24x + 16.7; r = 0.92, P < 0.001; Figure 2b).
Wing size showed no correlation with the water content of the resource but was positively related to resource pH (y = 1.51x + 67.7; r = 0.64, P < 0.05; Figure 2c).
The occurrence of an ‘optimal’ resource water content has
been reported for a number of dipteran larvae.
Fatchurochim et al. (1989) reported
that survival of various species of muscid larvae was zero in manure with
extreme high (>80%) or low (20%) water contents and was optimal in the
40%-70% moisture range. Bay et
al. (1969) found survival of
the face fly was prohibited in dung with a moisture content less than 66%, and Hodge and Wilson (1997) found that the survival of D. melanogaster and D. hydei in laboratory media was lower at the edges of the range of resource water contents they used (66% to 86%).
At very high liquidities, larval feeding tunnels in the resource may tend to collapse more readily, and small first instar larvae may be prone to drowning. The low survival in highly liquid resources may explain why Drosophila larvae tend to avoid very watery tissue by modifying their feeding position (McCoy, 1962; Arthur and Cassey, 1992). At low water contents, simple desiccation of the larvae may cause mortality, and David et al. (1983) also suggested that the osmotic stress of living in a very concentrated food resource may cause larval death.
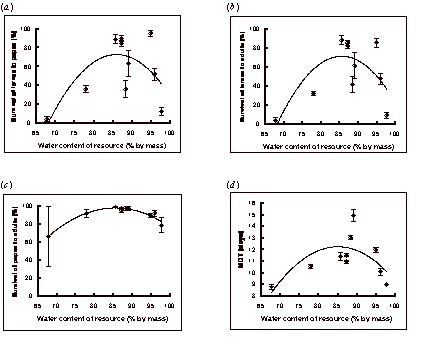
Figure 1. The effect of resource water content on (a) survival of larvae to pupae, (b) survival of larvae to adults, (c) survival of pupae to adults, and (d) MDT (mean ± SE).
A lowering of pupal viability at the extremes of resource moisture content has been reported for other Diptera. Barnard and Harris (1992) reported that pupal viability of Musca domestica was lowest in the wettest manure they examined, whereas Cook et al. (1980) found that in dry habitats muscid pupae were smaller and more prone to mortality.
Pupal survival was highest in the more acidic resources. This is surprising given that low pH gave a reduced body size in the emerged adults, suggesting these flies emerged from smaller pupae, which are often considered less viable. Bridges and Darby (1933) found that the thickness of the pupal case was reduced in Drosophila reared on acidic media, again suggesting a negative effect of high acidity on the pupae. Acidity affects the distance from the resource at which the larvae pupate (Hodge and Caslaw, 1998), and it is possible that this behavioural adaptation compensates in some way.
|
The effect of water
content on development time seems to disagree with other reports.
An extension of development time has been found on dilute resources,
possibly because feeding efficiency is reduced by the low viscosity (Sang,
1949; David et al., 1983). Posch
(1971) gave the opposite view when suggesting that development time might
be extended on more viscose media, as the larvae expend more energy
pushing through it. These arguments would suggest that development
time would be longest on the driest and wettest resources, whereas in the
present study the opposite situation occurred. MDT was shortest on the driest and wettest resources. In Drosophila, rapid development occurs not only when the larval
habitat is favourable, but can also occur if a habitat is inferior or on the
verge of disappearing, where the larvae pupate early in a last-ditch effort
to produce adult flies. The latter
situation seems to be occurring in the current experiment. The habitats which produced the most rapid
larval development (avocado and lemon) were those with the lowest survival,
suggesting conditions were not favourable and that the larvae pupated early.
An indication that the minimum viable pupation weight was not always
achieved is provided by the pupal viability being lowest on these two resources.
On more acidic resources, development time was extended and the adults were smaller. Other investigations using laboratory culture media have also suggested that development is prolonged on more acidic resources (Burdick and Bell, 1954; Hodge et al., 1996), and Hodge (1995) also found a decrease in wing length in both D. melanogaster and D. hydei raised on acidic media. As observed previously (Hodge et al., 1996), the larvae on the most acidic resources appeared listless, were struggling to gain body weight, and seemed to be experiencing nutritional problems. It can be speculated that low pH might affect the populations of yeasts on which the larvae feed and/or the ability of the larvae to digest their food.
In conclusion, although Drosophila larvae primarily obtain their nutrition from microorganisms present in fermenting vegetable matter, the physical and chemical properties of their habitat can also affect their development. It would appear that at extremes of resource moisture content, the development of larvae is barred, whereas resource acidity somehow affects larval nutrition. The mechanisms behind these effects require clarification. Also, many of the relationships found in this investigation, especially with water content of the resource, were statistically weak and repetition of the experiment, using a greater number of resources, is warranted.
References: Arthur, W., 1986, Phil. Trans. Roy. Soc., B. 313: 471-508; Arthur, W., and S. Cassey 1992, Ecol. Ent. 17: 354-358; Bay, D.E., C.W. Pitts, and G. Ward 1969, J. Econ. Ent. 62: 41-44; Bridges, C.B., and H.H. Darby 1933, Amer. Nat. 67: 437-472; Burdick, A.B., and A.E. Bell 1954, Dros. Inf. Serv. 28: 112-113; Caslaw, P., and S. Hodge 1999, Dros. Inf. Serv. 82: 46-49; Chan, H.T., and E.B. Jang 1995, J. Econ. Ent. 88: 569-573; Cook, I.M., A.V. Spain, and D.F. Sinclair 1980, Aust. J. Zool. 28: 547-52; David, J.R., R. Allemand, J. Van Herrewege, and Y. Cohet 1983, In: The Genetics and Biology of Drosophila, vol 3d. (Ashburner, M., H.L. Carson, and J.N. Thompson, jr., eds.). London, Academic Press; Goldat, S.J., and V.N. Baliaieva 1935, Zeitschrift fur Biologie 4: 379-384; Fatchurochim, S., C.J. Geden, and R.C. Axtell 1989, J. Ent. Sci. 24: 224-231; Hodge, S., 1995, Interspecific facilitation in Drosophila systems. Unpublished Ph.D. thesis. University of Sunderland, UK; Hodge, S., R. Campbell-Smith, and N. Wilson 1996, Entomologist 115: 129-139; Hodge, S., and P. Caslaw 1998, J. Insect Behav. 11: 47-57; Hodge, S., and P. Mitchell 1998, Dros. Inf. Serv. 81: 131-133; Hodge, S., and N. Wilson 1997, Entomologist 116: 93-103; McCoy, C.E., 1962, J. Econ. Ent. 55: 978-985; Morgan, R.O., and C.D. Schmidt 1966, J. Econ. Ent. 59: 222-223; Posch, N.A., 1971, Dros. Inf. Serv. 46: 56-57; Sameoto, D.D., and R.S. Miller 1968, Ecology 49: 177-180; Sang, J.H., 1949, Phys. Zool. 22: 183-202.