
De Toni, D.C. , C. Beuren Araújo, N.B. Morales, and V.L.S. Valente. 2001. Reference photomap of the salivary gland polytene chromosomes of Drosophila neocardini (Streisinger, 1946). Dros. Inf. Serv. 84:88-91.
|
|
|
|||
|
|
||||
Reference photomap of the salivary gland polytene chromosomes of Drosophila neocardini (Streisinger, 1946).
De Toni, D.C. 1, C. Beuren Araújo2, N.B. Morales2, and V.L.S. Valente1,2. 1 Programa de Pós Graduação em Biologia Animal-UFRGS; 2 Departamento de Genética, Instituto de Biociências-UFRGS: Universidade Federal do Rio Grande do Sul; danidetoni@bol.com.br.
Drosophila neocardini belongs to the cardini group, with D. polymorpha and D. cardinoides, and others common in the Neotropical region (Val et
al., 1981). These species, particularly,
D. neocardini and D. polymorpha,
are extremely similar with respect to their morphology and ecological requirements
(Rohde and Valente, 1996a). The specific differentiation is made through the
analysis of the internal male genitalia and and the pattern of abdominal pigmentation. In the middle of the sixth tergite of
D. neocardini, there is a black
square, a pattern of abdominal pigmentation that does not occur in the other
species of the cardini group
(Freire-Maia and Pavan, 1949).
Although these three Neotropical species share some environments
in nature, D. cardinoides is more frequently found in drier places, whereas D. polymorpha and D. neocardini are typical forest flies. D. neocardini, however, is much less frequent than D. polymorpha
(De Toni and Hofmann, 1995). As a starting point to study the inversion
polymorphism in D. neocardini, we constructed a reference photomap of the polytene chromosomes of third
instar larvae salivary glands. The
slide preparations were made through the method of Ashburner (1967).
At least 258 individuals (about three nuclei per gland) from 7 different
places (Campeche Island: 27º41’S and 48º28’W; Sertão da Lagoa do Peri: S 27˚ 45.234’ and W 48˚ 32.576’; State
Park of Serra do Tabuleiro: S 27˚ 44.480’ and W 48˚48.436’;
Arvoredo Island: S 27º 35.268’ and W 48º 28.329’; Lagoa da Conceição Hill:
S 27º 17.735’ and W 48º 21.437’; Ratones Grande Island: S 27º 28.584’
and W 48º 33.709’; Ratones Pequeno Island: S 27º29.687 and W 48º 33.967’,
all in the Brazilian state of Santa Catarina ) were analyzed and photomicrographed
to reach a consensus on the identity of the chromosomal elements.
The chromosomal complement of the salivary glands of
D. neocardini consists of 4 chromosome
pairs: submetacentric chromosomes II and III, the sexual pair (composed of
the acrocentric XX and the Y chromosome which is heterochromatic and not distinguishable
from the chromocenter), and the fourth, a dot pair.
This chromosomal complement was deduced by comparison of the polytene banding patterns of D. neocardini with those of the related species D. polymorpha and D. cardini (Rohde and Valente, 1996b).
Five chromosomal arms linked to the chromocenter can
be observed in the salivary gland squashes. The shorter arm is the left arm of chromosome II (IIL), followed
by X chromosome and the left arm of chromosome III (IIIL), which are both
equivalent in size. The right
arm of chromosome III (IIIR) and the right arm of chromosome II (IIR) are
the longest, respectively. In
Figure 1 the photomap of D. neocardini is
presented. The X chromosome (pair
1) was subdivided into 20 sections (from the tip to the base) and is distinguished
from the remaining chromosomes by the following characteristics: its terminal
part (section 1) stays permanently puffed during the third instar, and its
basal section (20) remains attached to a great portion of the chromocentric
heterochromatin. The left arm
of the chromosome II (IIL) was subdivided into sections beginning by the section
21 (tip) ending in the section
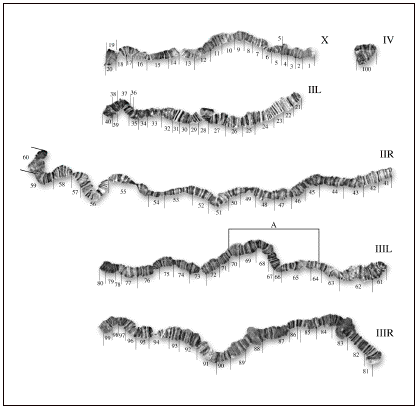
40 (base).
This chromosomal arm is relatively free of puffed bands in the third
instar larvae. The extremity of
section 21 presents the aspect of a “tip of a spatula”. The right arm of the chromosome II
(IIR), the longer one, was subdivided into sections numbered from 41 to 60. In the more proximal section (60), we observed a puff characteristic of this larval instar, whereas the remaining sections do not present constant puffs. The tip in section 41 has a straight aspect, showing eventually a sharp form.
The left arm of chromosome III (III L) is very short and was subdivided into sections numbered from 61 to 80. In the sections close to the tip, between the proximal median part of section 61 and the distal median of 62, there are several bands and interbands with approximately the same width, characteristic of this chromosome. The remaining sections present neither particular “landmarks” nor constant or characteristic puffs, and the tip (in section 61) always shows the same straight aspect, similar to that of section 41 of the IIR chromosomal arm. In this chromosomal arm, we frequently registered the occurrence of a heterozygous paracentric inversion involving the distal part of section 65 and the proximal one of section 70, here called IIILA, whose proposed breaking points are presented in the Figure 1. Figure 2 corresponds to the aspect of the inversion IIILA in heterozygote larvae.
The right arm of chromosome III (III R) is very long. It was subdivided into sections numbered from 81 to 99. Its tip in section 81 has a spatula-like form. The distal part of section 83 and of section 88 are frequently puffed in this developmental phase. Finally, the small chromosome IV, more frequently attached to the chromocenter, and comprehend the section 100.
|
Figure 2. Aspect of the paracentric inversion
IIILA in heterozygous state found in samples of Drosophila neocardini of Santa Catarina, Brazilian South. Bar = 10m. |
The sections of the polytene chromosomes of D. neocardini were compared to the photomap of D. cardinoides and D. polymorpha to determine areas of apparent homology and was performed mainly based in the photomap of D. cardinoides (Rohde and Valente, 1996b), because the chromosomes of these two species were more similar between them than with those of D. polymorpha with respect to their banding patterns, especially considering the length of the sections, of the sections, and the width and aspect of bands or groups of bands (“marker bands”). Sometimes we were not able to establish a clear homology between sections of polytene chromosomes of the cardini group species studied. In these cases, we subdivided the sections in the chromosomes of D. neocardini, maintaining lengths approximately similar to those correspondent in the related species. When the genetic content of these species groups are revealed in future studies, this first photomap, probably could be considerably improved.
Acknowledgments: The authors recognized Dr. Paulo R.P. Hofmann and Universidade Federal de Santa Catarina for all support to the realization of this study, to Dr. Claudia Rohde, for the help with the identification of the chromosomes and to PhD. student Jennifer Brisson for translating help. This study was performed with grants and fellowships of the following Brazilian agencies: CNPq, FAPERGS, PROPESQ-UFRGS.
References: Ashburner, M., 1967, Chromosoma 27: 47-63; De Toni, D.C., and P.R.P. Hofmann, 1995, Revta. Bras. Biol., 55(3): 347-350; Freire-Maia, N., and C. Pavan 1949, Cultus 1(5): 1-71; Rohde, C., and V.L.S. Valente 1996a, Revta. Bras. Ent. 40(1): 75-79; Rohde, C., and V.L.S. Valente 1996b, Braz. J. Genet. 19(1): 27-32; Val, F.C., C.R. Vilela, and M.D. Marques 1981, In: The Genetics and Biology of Drosophila. (Ashburner, M., H.L. Carson, and J.N. Thompson, jr., eds.), 3a: 123-168. Academic Press, London.