
Kiger, John A., Jr., and Christopher I. Ho. 2001. P repressor as a tool to analyze GAL4/UAS enhancer trap phenotypes in Drosophila melanogaster. Dros. Inf. Serv. 84: 188-199.
|
|
|
|||
|
|
||||
.
P repressor as a tool to analyze GAL4/UAS enhancer trap phenotypes in Drosophila melanogaster.
Kiger, John A., Jr., and Christopher I. Ho. Molecular and Cellular Biology, University of California, 1 Shields Avenue, Davis, CA 95616.
Abstract
P Repressor suppresses expression
of UAS transgenes. Using
mutant P elements that make
Repressor but cannot make Transposase, we demonstrate the utility of Repressor
for analysis of GAL4/UAS phenotypes
in three paradigms. 1. Mitotic
crossing over was used to remove a mutant P element from clones to generate genetic and phenotypic
mosaics. We investigated the
cellular specificity of wing phenotypes produced by ectopic expression of
Protein kinase A catalytic subunit (PKAc) in wing hemocytes and in wing epithelial
cells. 2. Repressor permits the
survival of adults carrying lethal combinations of GAL4 and UAS
P elements. Balanced lethal stocks of mutant P element and lethal GAL4/UAS combinations have been created that can be used in
genetic screens to identify genes that physiologically interact with the GAL4/UAS combination to suppress lethality. 3. In the study of GAL4/UAS phenotypes that are pleiotropic or are modified by
other aspects of the genotype, a mutant P element can be used to demonstrate that the observed effects are indeed
caused by GAL4-driven expression of the UAS transgene rather than by some genetic interaction created
by the genetic background.
Introduction
The P element-based GAL4/UAS system developed by Brand and Perrimon (1993) has become a standard tool to target gene expression to specific cells in Drosophila melanogaster in order to observe in vivo effects on the phenotype. In the course of studies using this system, we found that wildtype P elements suppress GAL4/UAS phenotypes. It has been shown for P strains derived from natural populations that P Repressor partially blocks transcription of P-LACZ fusion genes by binding to the P promoter (Lemaitre and Coen, 1991). Repression of the P promoter by Repressor is not complete, however, since a significant level of transcription is required to maintain Repressor synthesis (Roche et al., 1995). Like LAC-Z transcription, GAL4 transcription is initiated from the P promoter in GAL4 transgenes (Brand and Perrimon, 1993), suggesting a mechanism for at least a part of the suppression we observe. P repressor has also been shown to suppress transcription from heterologous promoters carried in P elements by a chromatin-based mechanism involving Polycomb-group genes (Roche et al., 1995; Roche and Rio, 1998). Therefore, it is possible that suppression of GAL4/UAS transgene phenotypes is due to synergism in the repression of transcription from both GAL4 and UAS P elements.
Here we investigate the potential utility of P Repressor as a means of genetically and phenotypically manipulating GAL4/UAS combinations. For such studies it would be highly desirable (essential in some cases) that these P elements do not change their chromosomal locations due to the action of P Transposase. This consideration led us to investigate the properties of mutant P elements that make Repressor but cannot make Transposase (Karess and Rubin, 1984; Misra and Rio, 1990; Rio, 1991). We have tested two such mutant P elements in three paradigms and found them to perform as useful tools for the analysis of GAL4/UAS combinations.
First, we show that P Repressor turns over sufficiently rapidly to be used to create genetic mosaics enabling clonal analyses of GAL4/UAS phenotypes. Mitotic crossing over has been used to investigate wing phenotypes produced by ectopic expression of Protein Kinase A catalytic subunit (PKAc). We show that wing phenotypes can be caused by ectopic PKAc expression in either wing epithelial cells or in wing hemocytes and relate these phenotypes to previous studies. These results provide novel evidence that wing hemocytes represent a unique population of hemocytes, one that is not recruited from the general circulation. The implications of this hemocyte population for wing morphogenesis are discussed
Second, P Repressor permits adults carrying lethal combinations of GAL4 and UAS P elements to survive. Balanced lethal stocks of mutant P and GAL4/UAS combinations are easily produced. These stocks can be used in genetic screens to identify genes that physiologically interact with the GAL4/UAS combination to suppress the lethal effect.
Third, in the study of GAL4/UAS
phenotypes that are pleiotropic or may be modified by other aspects of the
genotype, a mutant P element can be used to determine if the observed phenotypic
effects are caused by GAL4-driven expression of the UAS transgene rather than by some other genetic interaction
created in crossing strains with different genetic backgrounds.
Results
Suppression
of GAL4/UAS phenotypes by Wildtype P elements.
Expression of UAS-PKAc transgenes using the GAL4-30A enhancer-detector strain produces severely blistered or collapsed wings and melanotic growths that are frequently found in the head. These effects are caused by PKAc expression in hemocytes around the time of eclosion (Kiger et al., 2001). To study these effects we have used a chromosome carrying both the GAL4-30A transgene and the UAS-PKAc 15.3 transgene. When flies carrying this chromosome are crossed to flies of interest, the GAL4-30A, UAS-PKAc 15.3 combination is transmitted from the first parent as a unit, allowing effects of genes or transgenes transmitted from the second parent to be assessed from the phenotype of progeny that receive both.
When GAL4-30A, UAS-PKAc 15.3 / CyO flies are crossed to flies of two different P strains carrying multiple P elements (Rio, 1991) the non-CyO progeny have a completely normal phenotype, i.e. the GAL4-30A, UAS-PKAc 15.3 phenotype is completely suppressed (data not shown). To study the effects of a single P element, we employed a chromosome carrying the cactBQ mutant, which was induced by P mobilization using the P strain Harwich (Daniel St Johnston, personal communication). This mutation is caused by insertion of a full length P element into cact exon 2(Bergmann et al., 1996). The cactBQ chromosome produces complete suppression of the GAL4-30A, UAS-PKAc 15.3 phenotype (Figure 1A). Attempts to prove identity of the cactBQ mutation and the suppressor by mobilization of the P element and selection for reversion of cactBQ were not successful, necessitating mapping of the suppressor locus. The suppressor maps to the location of cactBQ as discussed in MATERIALS AND METHODS. This P element is evidently transcriptionally active because we have found that the cactBQ chromosome can mobilize a UAS transgene (data not shown).
To test the phenotypic specificity of suppression, we examined the effects of the cactBQ chromosome on other combinations of GAL4 enhancer-detector strains and UAS-transgenes. In addition to expression in hemocytes, GAL4-30A is strongly expressed in the salivary glands of 3rd instar larvae as shown using a UAS-GFP transgene. The cactBQchromosome strongly reduces the level of GFP
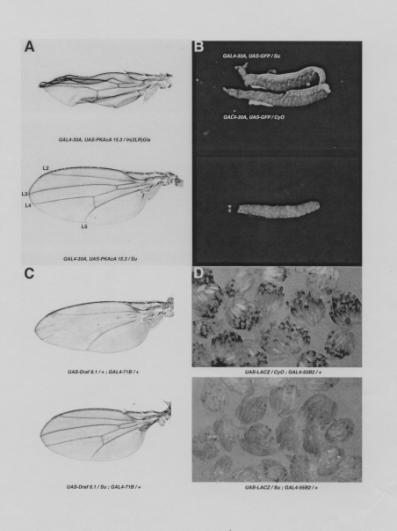 |
| Figure 1. The effects of
the cactBQ chromosome, designated Su,
on the phenotypes of different GAL4/UAS combinations. (A) The collapsed wing phenotype produced by GAL4-30A
and UAS-PKAc 15.3. Indicated
in the bottom panel are the longitudinal vein designations of the normal
wing. (B) Fluorescence in
salivary glands produced by GAL4-30A, and UAS-GFP. The top panel shows salivary glands illuminated
from the side using tungsten light. The bottom panel shows the same salivary glands using epifluorescence.
(C) The wing phenotype produced by UAS-Draf 6.1 and
GAL4-71B. (D) X-gal staining of LACZ activity in ovarian follicle cells produced
by UAS-LACZ and GAL4-55B2. |
Mosaic
analyses of wing phenotypes caused by ectopic PKAc expression using the SalI
mutant Repressor.
The wildtype P element carries a single
transcription unit whose primary transcript can be spliced to produce 87kD
Transposase or a C-terminally truncated, 66kD Repressor (Rio, 1991). The mutant
P[ry+; Sal I] element contains
a frameshift mutation near the middle of exon 4 of the transcription unit,
causing polypeptide termination 10 amino acids downstream of that site, inactivating
Transposase but not Repressor (Karess, and Rubin, 1984; Robertson, and Engels,
1989). P[ry+;
Sal I] 89D suppresses the GAL4-30A, UAS-PKAc 15.3 phenotype as effectively as the cactBQP element (data not shown). We tested the ability of the SalI Repressor to generate mosaics following mitotic crossing
over as follows. Females of genotype
y, w, P[ry+; hs-FLP] ; FRT82B
/ TM3, Sb were crossed to males of genotype
y, w / Y; GAL4-30A, UAS-PKAc 15.3 / +; FRT82B, SalI 89D, y+/ +. Larvae
were, or were not, heat shocked as described in Materials and Methods. The incidence of y+ progeny adults, homozygous or heterozygous for FRT82B, exhibiting
one or more wing blisters is recorded in Table 1. The data show that the presence of a wing blister requires
both a heat shock during larval development and homozygosity for FRT82B, demonstrating that the blisters must be the result
of mitotic crossing over. The
blisters in general are quite large and tend to be round, whereas mitotic
clones induced in wing epithelial cells are usually smaller and elongated
along the proximal-distal axis (Garcia-Bellido and Merriam, 1971). One of the blistered wings is shown in
Figure 2A. The blister encompasses
a large portion of the anterior wing margin where the large bristles can be
seen to be y+. The presence of a nearby y bristle is evidence of mitotic recombination having
occurred in epithelial cells that produced this wing. The y phenotype
cannot be scored reliably in the wing blade cuticle or the wing hairs, however.
Table 1. hsFLP-Induced mitotic recombination in
P[ry+; SalI] / + flies.
|
Gal4/UAS Combination |
Incidence of Flies with Abnormal
Wings |
||
|
Heat Shock |
No Heat Shock |
Heat Shock |
|
|
FRT/FRT |
FRT/FRT |
FRT/ + |
|
|
GAL4-30A,
UAS-PKAc 15.3 |
47/147 |
0/132 |
0/133 |
UAS-PKAcF 1.1;
Gal4-71B
|
48/108
|
0/93
|
|
|
|
|||
|
Figure 2. Mosaic blistered wing phenotypes produced
by heat shock induced mitotic recombination. (A) Wing of a fly of genotype y,
w, P[ry+; hs-FLP]/ y, w ;
GAL4-30A, UAS-PKAc 15.3 / + ; 82B, SalI, y+/ 82B. y is easily scored
only in the large bristles along the anterior margin of the wing. The very large bristle (arrow) is y. Those
bristles encompassed by the large blister are y+. (B) Wing of a fly of genotype w,
P[ry+; hs-FLP] 9F, f36a / y, w, f36a; GAL4-30A, UAS-PKAc 15.3 / + ; 82B, SalI, y+, f+/
82B. A blister (area bounded by creases marked “c”)
encompasses a portion of L2.
A large clone of f36a hairs (identified to the right by “*”)
runs through the blister anterior (above) to L2 without crossing the
vein. Most of the hairs
posterior (below) to L2 are f+. |
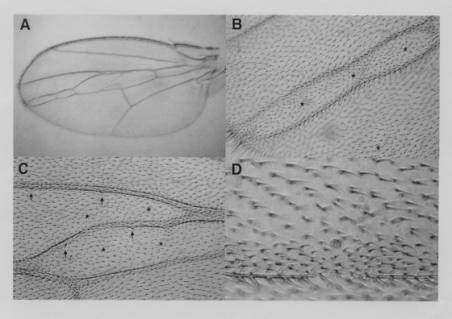
We then constructed stocks that allow clones in the wing blade to be marked with f36a. A wing showing such clones is shown in Figure 3A. Interestingly, only clones falling between L3 and L4 have phenotypic effects. Clones located elsewhere do not disturb the normal wing pattern. Figure 3B shows the distal portion of the wing in Figure 3A at higher magnification. In the region of the normal L4 vein which is missing there is a large clone marked with f36a bounded on each side by ectopic veins that appear to be a mirror-image duplication. Most of these veins have f36a hairs on them except near the distal ends. These veins extend to the wing margin where socketed f+ bristles can be seen that are characteristic of L2 or L3 but not of L4. Figure 3C shows an ectopic vein in another wing joining L3 and L4. The ectopic vein is clearly of the L3 type because it has sensilla that are characteristic only of L3. Figure 3D shows a clone of f36a hairs along the anterior edge of L4. Within the clone is an ectopic sensillum characteristic of L3 and curiously nearby there is a small gap in L4.
Genetic
manipulation of lethal GAL4/UAS combinations using the 66k mutant Repressor.
We have shown in the above section how P[ry+; Sal I] 89D can be used to suppress the lethality of the GAL4-71B and UAS-PKAcF 1.1 combination in creating a genotype that permits mosaic analysis of this GAL4/UAS phenotype. We have tested the utility of the mutant P[ry+; 66k] inserted in the chromosome II balancer CyO for suppressing lethality caused by the PKAc inhibitor PKIF in the combination GAL4-PP3, UAS-PKIF 5-1. These GAL4 and UAS transgenes are linked to chromosome III (Kiger et al., 1999). P[ry+; 66k] is a deletion construct that removes the downstream half of intron 3, including the 3' splice site, and most of exon 4 of the P transcription unit so that only the normal 66kD Repressor protein is produced (Misra and Rio, 1990). Males of genotype w / Y ; CyO, P[ry+, 66k] / + ; GAL4-PP3 / + were crossed to females of genotype y, w ; UAS-PKIF 5-1. The expected progeny classes and (numbers recovered) were: Cy / + ; UAS / + (118); + / + ; UAS / + (114); Cy / + ; UAS / GAL4 (97); + / + ; UAS / GAL4 (0). Thus, suppression of lethality is quite effective.
Females of genotype y, w / w ;
CyO, P[ry+, 66k] / + ;
UAS-PKIF 5-1 / GAL4-PP3 were then
crossed to males of genotype w / Y
and progeny males of genotype w / Y; CyO, P[ry+, 66k] / + ; GAL4-PP3 , UAS-PKIF 5-1/ + selected on the basis of eye color. Single males were
then used to produce balanced stocks of the genotype w ; CyO, P[ry+, 66k] / + ; GAL4-PP3 , UAS-PKIF 5-1/ TM3. We
also have maintained this GAL4/UAS
combination in a stock in which the females are y, w ; SalI 89D,
y+ and the males are y, w ; GAL4-PP3, UAS-PKIF 5-1/ SalI 89D, y+. The fact
that crossing over does not occur in males preserves the combination. Such
a stock of course needs selection every generation so that only y,
w ; SalI 89D, y+ females breed
the next generation. Stocks such
as these can be used to screen flies for dominant phenotype-specific suppressors
of the GAL4/UAS combination
by a single cross.
Analysis
of GAL4/UAS phenotypes as a function of the genetic background.
GAL4/UAS combinations often exhibit quite pleiotropic effects.
For example, in addition to wing blisters and melanotic growths in
the head, described above, flies carrying the GAL4-30A,
UAS-PKAc 15.3 chromosome exhibit
a developmental delay of about 2 days compared to sibs that do not carry this
combination. One or another of these pleiotropic phenotypes might be caused
by mutations created by the insertion of one or both of these P elements into the chromosome or by some other aspect
of the genetic background. Introducing
P[ry+; SalI]
into the genetic background causes suppression
of all three phenotypes, and its subsequent removal from the genetic background
restores all three phenotypes. Thus, the pleitropy of GAL4-30A, UAS-PKAc 15.3 must be caused by
GAL4-driven expression of the UAS
transgene. Quantitative data
on suppression of the wing blister and melanotic growth phenotypes by two
different P[ry+;
SalI] insertions are given in
Table 2.
 |
|
Figure 3. Mosaic wing veination phenotypes produce by heat shock induced mitotic recombination in flies of genotype w, P[ry+; hs-FLP]9F, f36a / y, w, f36a; UAS-PKAcF 1.1 / +; GAL4-71B, 82B, SalI, y+, f+/ 82B. (A) Ectopic vein formation in the intervein region between L3 and L4. (B) Distal portion of the wing in A at higher magnification centered on the L4 region. Note the large clone of f36a hairs (marked by “*”) filling the region between the doubled veins and the f+ socketed large bristles where the doubled veins meet the margin of the wing. Socketed bristles are characteristic of the ends of L2 and L3 but not of L4. Note also a region of f36a hairs in the lower right corner (marked by “*”) that has no effect on the pattern of the wing. (C) In another wing, an ectopic vein joins L3 and L4. Note the f36a hairs on both sides of the ectopic vein (marked by “*”) and the sensilla (arrows) on the normal L3 and on the ectopic vein. Normally only L3 has such sensilla. (D) Another wing at higher magnification showing a clone of f36a hairs along the anterior edge of L4. Note that an ectopic sensillum characteristic of L3 has formed within the clone and that posterior to the sensillum there is a gap in L4.
|
|
|
|||||||||||||||||||||||||||||||||
Discussion
P
Repressor as a suppressor of UAS transgenes.
The multiple P elements in P strains and P[ry+; Sal I] 89D repress transcription of P-LACZ fusion genes (Lemaitre and Coen, 1991). Quantitative measurements of LACZ activity in larvae show a 40% reduction by P[ry+; Sal I] 89D and 65% reduction by Harwich P strain: relative values reported are 1.5 [Harwich P strain], 2.6 [P[ry+; Sal I] 89D] and 4.2 [P[ry+; RI] 86A, inactive control]. While we have not made quantitative measurements, based on the effect of cactBQ on expression of the UAS-LACZ or UAS-GFP transgenes (Figure 1B and 1D), it appears that we are seeing more than a 65% reduction in expression of these GAL4/UAS combinations. However, suppression is obviously not complete. Table 2 showing the frequency of flies exhibiting complete suppression of wing blistering and melanotic growths demonstrates greater than 99% suppression. The difference between these examples is certainly due to the different natures of the assays. The level of LACZ activity or of GFP fluorescence is related more or less directly to the level of transcription and translation of mRNA. On the other hand, morphological phenotypes are more likely to be subject to threshold effects.
We have found GAL4/UAS combinations
that cannot be suppressed with the two mutant P elements we have tested.
For example, we were unable to suppress lethality and create a balanced
stock of the combination GAL4-JW1
and UAS-PKIF 1-1 using P[ry+;
Sal I] 89D. The
usefulness of mutant P elements
must be tested on a case-by-case basis.
Suppression of the phenotypic effects of UAS transgenes expressing highly toxic products may be
difficult to achieve due to a low threshold of tolerance. The use of a stronger
promoter to express a Repressor cDNA could be a solution to this problem. Nevertheless, we have demonstrated the
usefulness of mutant P elements
in particular cases in three different experimental paradigms. Lee and Luo (1999) have used Gal 80 in
a similar manner to our use of Repressor to carry out mosaic analyses. The two systems should complement each
other in the Drosophila toolbox.
Mosaic
analysis of the GAL4-30A, UAS-PKAc 15.3
wing phenotype.
We previously presented evidence that the wing blister phenotype of
GAL4-30A, UAS-PKAc 15.3 is due to expression
of PKAc in hemocytes in the wing at the time of, or just prior to, wing expansion,
where their role may be to synthesize extracellular matrix that binds the
wing surfaces together (Kiger et al.,
2001). Because a large population of hemocytes in the wing had not been previously
described, we have sought ways to confirm that wing blisters are not caused
by expression in the wing epithelium which secretes the cuticle that forms
the wing surfaces. To do so,
we have induced mitotic recombination in 1st instar larval cells
soon after hatching. One would
think that at the time of wing morphogenesis, hemocytes derived from a mitotically
recombined 1st instar larval hemocyte precursor might have become
diluted in the larger population of hemocytes derived from non-recombined
hemocyte precursors. However,
we reasoned that wing hemocytes might not be part of the general circulation.
Milner and Muir (1987) noted that cultured wing discs have adhering
hemocytes that become internalized when the disc evaginates so that the hemocytes
are then found in the lumen of the wing.
Division of adhering hemocytes prior to evagination and/or division
of hemocytes in the lumen during the period of wing growth after evagination
(Murray, et al., 1995) could
produce a clone of hemocytes, over a substantial area of the wing blade, that
fails to synthesize extracellular matrix.
The observations of wing blisters we present here are evidence that
hemocytes are resident in the wing and not part of the circulating hemocyte
population. Studies of DNA replication in the developing
wing during the first 24 hr following pupariation have not identified any
large population of cells other than epithelial cells (Schubiger and Palka,
1987). Expansion of the hemocyte
population must occur after 24 hr following pupariation. Understanding of
wing morphogenesis will require consideration of when and how these two tissues
interact.
Mosaic
analysis of the UAS-PKAcF 1.1; GAL4-71B wing phenotype.
Expression of PKAc in the wing epithelium does not cause wing blisters, in contrast to the effects of PKAc discussed above. Expression of PKAc in the wing epithelium produces an effect only in clones located in the wing blade between L3 and L4, where ectopic development of L3 vein material occurs. These effects can be explained by inactivation of the transcription factor Ci-155 (Aza-Blanc and Kornberg, 1999) due to PKAc phosphorylation (Wang et al., 1999; Kiger and O'Shea, 2001) and consequent failure to induce Patched, Knot and Vein activity.
The region between L3 and L4 is the central organizer of the wing specified by the Hedgehog signal emanating from the posterior compartment (Bier, 2000; Mohler et al., 2000). Hedgehog regulates Ci-155 to activate transcription in the organizer region of genes that include patched, knot and vein. Knot is a transcription factor that represses vein development in the blade region between L3 and L4. Vein is a ligand that diffuses beyond the organizer region and induces vein development in cells just outside of the region of Knot expression. Thus, the vein that forms in the anterior compartment is L3 and that that forms in the posterior compartment is L4. Clones of knot mutant cells in the wing blade form ectopic vein material but only when the clone is between L3 and L4, just as we see for ectopic PKAc expression (Nestoras et al., 1997).
In Figure 3B, failure of L4 to form could be due to failure of the
cells in the clone to produce the Vein ligand that induces L4. The anterior of the mirror-image pair of veins forms because
Knot is not expressed in the cells that initiate vein formation. They receive Vein from the cells outside
the clone. Being in the anterior
compartment, it is L3 vein that forms. The ability of vein material to induce vein formation in nearby
cells could explain why the cells at the wing margin forming socketed L3-type
bristles are outside the clone (Garcia-Bellido, 1977; Mohler et al., 2000). It
is less clear why the posterior of the two mirror-image veins form.
However, similar effects are produced by loss of either Patched or
Knot activity in clones (Phillips et al.,
1990; Nestoras et al., 1997). In
Figure 3D, the clone anterior to L4 is smaller than that in Figure 3B and
causes only a small gap in L4, suggesting that Vein may be able to diffuse
through most of the clone to reach the posterior compartment.
Materials
and Methods
Strains
The UAS strains employed have been described previously (Kiger et al., 1999) with the exception of the UAS-Draf strain, that was created by standard means using a wild type cDNA provided by Klaus Dncker. GAL4-30A and GAL4-71B are described in Brand and Perrimon (1993) with the corrigendum that their identities are reversed in that paper. GAL4-55B2 is described in Brand and Perrimon (1994). All GAL4 and UAS transgenes carry the mini-w+ gene and are in a w background so that the presence of each transgene can be identified by an eye color phenotype that is more dilute than that caused by w+. Frequently, the presence of more than one mini-w+ transgene can be detected by the additivity of the eye colors produced, making it possible to carry out crosses between heterozygous transgenic strains and to identify all progeny classes unequivocally.
The P strains employed, derived from natural populations of D. melanogaster located in Northern California at Auburn and Wolfskill, were provided by M. M. Green. The cactBQ stock was donated by Ruth Steward. The P[ry+; Sal I] 3D andP[ry+; Sal I] 89D strains were obtained from the Bloomington Stock Center and William Engels, respectively. P[ry+; SalI] 3D is inserted into the diminutive gene and is also known as dmP0. Strains carrying P[ry+; 66k] were obtained from Donald Rio.
|
Culture conditions and heat shock.
All crosses were carried out at 25ºC.
For heat shock experiments flies were allowed to deposit eggs in food
vials for 24 hours. After 24
hours the vial was submerged in a water bath at 38ºC for either one or
two hours and then returned to 25ºC.
Microscopy and staining.
Ovaries were stained for LACZ activity using Xgal as described by Brand
and Perrimon (1994). Ovaries
and salivary glands were photographed using a Leica MZ FLIII fluorescence
stereomicroscope. Wings were mounted as described in Kiger
et al. (1999) and photographed (Figure
1) with a dissecting microscope (Zeiss, Jena, Germany) or (Figures 2 and 3)
a Zeiss Axioplan microscope equipped with a Kodak Photomicrography System
MDS290.
Mapping
the suppressor locus in the cactBQ chromosome.
The suppressor locus (Su), present on a chromosome carrying the known mutants b cactBQ pr cn, was mapped initially using a chromosome carrying six dominant markers, Sp Bl Lrm Bc Pu2 PinB. Found to be located on the left arm of chromosome II, Su was recovered in a recombinant chromosome carrying Sp b cactBQ pr cn. Next females of genotype (Su) Sp b cactBQ pr cn / + were crossed to males of genotype GAL4-30A, UAS-PKAc 15.3 / CyO, Roi cn. Preliminary analysis of the progeny indicated that Su was located between Sp and cn. With this knowledge, progeny with crossovers between Sp and cn were selected over CyO, Roi cn and crossed individually to In(2L) dlT, b dlT pr cn sca / CyO. From this cross, balanced stocks containing individual crossover chromosomes were established, and progeny carrying each crossover chromosome and In(2L) dlT were scored for b and pr ; female progeny were tested for fertility to determine the cact genotype. Females of genotype cactBQ/ In(2L) dlT are sterile (Roth et al., 1991). Males carrying each crossover chromosome then were crossed to females of genotype GAL4-30A, UAS-PKAc / CyO, Roi cn, and GAL4-30A, UAS-PKAc 15.3 progeny were scored for Su.
Analysis of 331 crossover chromosomes showed that Su
is located, as is the cact gene,
between b and pr (a distance
of 6.0 centimorgans). Of these crossovers, 52 single crossovers fell between b and pr
and failed to separate Su from
cactBQ. However,
4 triple crossovers separating the two were recovered, 3 moved Su to the b+ pr+ chromosome (Sp Su) and 1
moved cactBQ to the
b+ pr+ chromosome (Sp cactBQ). These unusual events may
be causally related to the presence of the P element. Thus,
cactBQ and Su
are very closely linked.
Acknowledgments: We thank M. M. Green for critically reading this manuscript. This work was supported by funds of the Agricultural Experiment Station at UC Davis.
References: Aza-Blanc, P. and Kornberg, T. B., 1999, Ci, a complex transducer of the Hedgehog signal. Trends in Genetics 15: 458-462; Bergmann, A., Stein, D., Geisler, R., Hagenmaier, S., Schmid, B., Fernandez, N., Schnell, B. and Nnsslein-Volhard, C., 1996, A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech. Dev. 60: 109-123; Bier, E., 2000, Drawing lines in the Drosophila wing: initiation of wing vein development. Curr. Opin. Gen. and Dev. 10: 393-398; Brand, A. H. and Perrimon, N., 1993, Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415; Brand, A. H. and Perrimon, N., 1994, Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes and Dev. 8: 629-639; Garcia-Bellido, A., 1977, Inductive mechanisms in the process of wing vein formation in Drosophila. Wilhelm Roux's Arch. Dev. Biol. 182: 93-106; Garcia-Bellido, A. and Merriam, J. R., 1971, Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev. Biol. 24: 61-87; Karess, R. E. and Rubin, G. M., 1984, Analysis of P transposable element functions in Drosophila. Cell 38: 135-146; Kiger, J. A., Jr., Eklund, J. L., Younger, S. H. and O'Kane, C. J., 1999, Transgenic inhibitors identify two roles for Protein Kinase A in Drosophila development. Genetics 152: 281-290; Kiger, J. A., Jr., Natzle, J. E. and Green, M. M., 2001, Hemocytes are essential for wing maturation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98: 10190-10195; Kiger, J. A., Jr. and O'Shea, C., 2001, Genetic evidence for a Protein kinase A/Cubitus interruptus complex that facilitates processing of Cubitus interruptus in Drosophila. Genetics 158: 1157-1166; Lee, T. and Luo, L., 1999, Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 451-461; Lemaitre, B. and Coen, D., 1991, P regulatory products repress in vivo the P promoter activity in P-lacZ fusion genes. Proc. Natl. Acad. Sci. USA 88: 4419-4423; Lindsley, D. L. and Zimm, G. G., 1992, The Genome of Drosophila melanogaster. Academic Press, San Diego; Milner, M. J. and Muir, J., 1987, The cell biology of Drosophila wing metamorphosis in vitro. Wilhelm Roux's Arch. Dev. Biol. 196: 191-201; Misra, S. and Rio, D. C., 1990, Cytotype control of Drosophila P element transposition: the 66 kd protein is a repressor of transposase activity. Cell 62: 269-284; Mohler, J., Seecoomar, M., Agarwal, S., Bier, E. and Hsai, J., 2000, Activation of knot (kn) specifies the 3-4 intervein region in the Drosophila wing. Development 127: 55-63; Murray, M. A., Fessler, L. I. and Palka, J., 1995, Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev. Biol. 168 150-165; Nestoras, K., Lee, H. and Mohler, J., 1997, Role of knot (kn) in wing patterning in Drosophila. Genetics 147: 1203-1212; Phillips, R. G., Roberts, I. A. H., Ingham, P. W. and Whittle, J. R. S., 1990, The Drosophila segment polarity gene patched is involved in a position-signalling mechanism in imaginal discs. Development 110: 105-114; Rio, D. C., 1991, Regulation of Drosophila P element transposition. Trends Genet. 7: 282-287; Robertson, H. M. and Engels, W. R., 1989, Modified P elements that mimic the P cytotype in Drosophila melanogaster. Genetics 123: 815-824; Roche, S. E. and Rio, D. C., 1998, Trans-silencing by P elements inserted in subtelomeric heterochromatin involves the Drosophila Polycomb group gene, Enhancer of zeste. Genetics 149: 1839-1855; Roche, S. E., Schiff, M. and Rio, D. C., 1995, P-element repressor autoregulation involves germ-line transcriptional repression and reduction of third intron splicing. Genes and Dev. 9: 1278-1288; Roth, S., Hiromi, Y., Godt, D. and Nnsslein-Volhard, C., 1991, cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development 112: 371-388; Schubiger, M. and Palka, J., 1987, Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev. Biol. 123: 145-153; Wang, G., Wang, B. and Jiang, J., 1999, Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes Dev. 13: 2828-2837; Xu, T. and Rubin, G. M., 1993, Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223-1237.