
Santos-Colares, M.C., B. Goñi2, and V.L.S. Valente. 2002. An improved technique for mitotic and meiotic chromosomes of Neotropical species of Drosophila. Dros. Inf. Serv. 85: 133-136.
|
|
|
|||
|
Next HTML file
|
|
|||
An improved technique for mitotic and meiotic chromosomes of Neotropical species of Drosophila.
Santos-Colares, M.C.1, B. Goñi2, and V.L.S. Valente1,3,4. 1Programa de Pós Graduação em Biologia Animal, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil; 2Facultad de Ciencias, Universidad de la Republica, Montevideo, Uruguay; 3Departamento de Genética, Instituto de Biociências, UFRGS. Caixa Postal 15053. CEP 91501-970. Porto Alegre, RS, Brazil. 4Emails: vera.gaiesky@ufrgs.br, vera.valente@bol.com.br
The cytological study of Drosophila chromosomes requires practical and simple techniques, to guarantee abundant, good-quality material. Several requirements should be taken into account, prior to the preparation of slides. Among them, we underline the care with laboratory environment conditions, since the results of the “air drying” technique change depending on the relative humidity of the air. This is particularly important when we try to apply the classic protocols (as those of Imai et al., 1977, 1988) to endemic species of Neotropical Drosophila in hot and humid places.
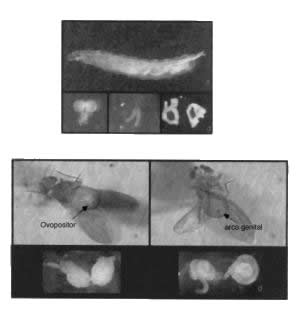 |
Figure 1. (a) Male larva of Drosophila willistoni; (b) larval brain ganglia dissected; (c) larval salivary gland dissected; (d) larval imaginal discs of gonads dissected. |
| Figure 2. Adults of Drosophila willistoni. (a) female; (b) male; (c) ovaries dissected; (d) testes dissected. |
The first step is to make the choice of the most suitable organs to
obtain the chromosome type desired (mitotic, meiotic, or polytene) and to
define the best part of the structure to be processed.
In Drosophila, the ideal organ
to allow the detection of mitotic figures are the brain ganglia of larvae
and pre-pupae (Figure 1). Good
quality metaphase plates and other mitotic phases can be obtained with relative
facility.
|
Figure 3. Schema adapted to that of Imai et
al. (1988), indicating the steps
of the modified protocol here presented to obtain mitotic and meiotic
chromosomes of Neotropical Drosophila. h.s. = hypotonic solution; or = organ; f.p. = filter paper. |
For the detection of meiotic chromosomes, imaginal discs of gonads of larvae and pre-pupae are the best material. In male and female adults, it is necessary to dissect testes and ovaries (Figure 2) and to find the parts of these structures with the best chances to provide divisions. In species polymorphic for paracentric inversions and other structural variations, the use of larvae and pre-pupae allows the comparison of the meiotic figures with the chromosomal arrangements present in the polytene cells of the salivary gland of the same individual. This is useful to evaluate the meiotic consequences of new chromosomal variants.
Some changes here presented were made after changing the basic technique of Imai et al. (1977, 1988), aiming to optimize the preparation of slides with Neotropical species, such as those from the Drosophila willistoni group under the room conditions commonly found in Brazil and other tropical countries, that are subject to similar climates. They are: (a) before their use, water-soap cleaned slides are stocked in an ethanol:sulfuric ether (3:1) solution up to several months. They are dried in paper tissue immediately before the preparation of the material, helping to eliminate residues that could form undesirable background; (b) the pre-treatment with colchicine was eliminated, and the time to dry the slides reduced to two hours, instead of the 24-h period used by Imai et al. (1988), before the staining with Giemsa. This procedure allows the analysis of the material in the same day, which could be important in the case of species that are difficult to rear in laboratory; (c) the slides should lie for a minimum time of 15 min in Giemsa 3%; (d) the excess of Giemsa is subsequently removed in running water, and then a gentle wiping of the opposite (back) surface of the slide using a paper tissue soaked in alcohol is suggested to improve the conditions of analysis of the material. This care helps again to avoid the occurrence of “background”.
The apparatus and material used are:
slides with depressions, fine forks, hypodermic needles, clean slides
(as referred to above), paper tissue, bottles for the fixative solutions,
and Pasteur pipettes. The solutions
used are as follows:
1. Hypotonic solution of sodium citrate (1%)
1 g dihydrated trisodium citrate (Na3C6H5O72H2O)
100 ml distilled H2O
2. Fixative solution I: acetic-ethanol (60%)
The fixative solutions should be prepared immediately before the processing of the slides: they cannot be stocked.
3 ml ethanol, 3 ml acetic acid, 4 ml distilled H2O
3. Fixative solution II: acetic-ethanol (100%)
2 ml ethanol, 2 ml acetic acid
4. Fixative solution III:
Glacial acetic acid
5. Phosphate buffer pH 6.8:
4.75 g dibasic sodium phosphate (Na2HPO4), 4.5
g monobasic potassium phosphate (KH2PO4), 1000 ml distilled
H2O
6. Giemsa - this solution can be used only two times - do not stock.
0.76 g Giemsa, 50 ml glycerol, 50 ml methanol
7. Staining solution (Giemsa 3%0
20 ml phosphate buffer, 77 ml distilled H2O, 3 ml Giemsa
Dissect the material in sodium citrate solution (see Figure 3) and:
|
Figure 4. (a) Mitotic metaphase of Drosophila
willistoni female larva; (b) meiotic chromosomes of a Drosophila
willistoni male larva. Bars = 10 mm. |
(a) transfer the material to a slide adding a drop of sodium citrate for at least 5 min;
(b) remove the excess citrate with the tip of a Pasteur pipette;
(c) place two drops of fixative solution I around the material, spreading with the tip of the hypodermic needle;
(d) place again two new drops of fixative solution I;
(e) observe the material under microscope and remove the excess of tissue;
(f) cut the material into small pieces using the hypodermic needle;
(g) add another drop of the fixative solution I;
(h - i) after the retraction of the fixative solution I, place small rolls of filter paper in the extremities of the slides to absorb the excess of liquid and add two drops of fixative solution II;
(j - l) take care for the fixation solution II to uniformly cover the material, allowing the spreading and sticking of the cells to the slide;
(m) after around 30 sec, add two drops of fixative solution III;
(n) wait for the complete retraction of fixative solution III and remove the excess. Leave the slide drying for at least 2 h at room temperature;
(o) stain the slide with Giemsa 3%, in phosphate buffer pH 6.8, for 15 min.
It is important to call attention to the fact that in brain ganglia preparations, mitotic figures (Figure 4a) are found in almost 100% of the slides, whereas meiotic male figures (Figure 4b) are detected in around 10% of the testes or imaginal discs processed.
Acknowledgments: This study was supported by the Brazilian Agencies CNPq, FAPERGS and PROPESQ-UFRGS (fellowships and grants) and by the PEDECIBA and CSIC Uruguayan Agencies (grants).
References: Imai, H.T., R.H. Crozier, and R.W. Taylor 1977, Chromosoma 59: 341-393; Imai, H.T., R.W. Taylor, M.W. Crosland, and R.H. Crozier 1988, Jpn. J. Genet. 63: 159-185.