
Bhattacharya, Sharmila, Robert Bowman, Frances Donovan, Beverly Girten, Esther Hill, Melissa Kirven-Brooks, Orlando Santos. 2002. Developing New Habitats for Life Science Experiments on the International Space Station. Dros. Inf. Serv. 85: 156-168.
|
|
|
|||
|
Next HTML file
|
|
|||
Developing New Habitats for Life Science
Experiments
on the International Space Station
Sharmila Bhattacharya, Robert Bowman, Frances Donovan, Beverly Girten, Esther Hill,
Melissa Kirven-Brooks,
Orlando Santos
NASA Ames Research Center
Moffett Field, CA
(This is an updated version of a paper reported in the
Publication of the American Institute of Aeronautics and Astronautics 2001,
#2001-4984)
Abstract
The Space Station Biological Research Project is developing habitats
to accommodate long duration experiments for a variety of research organisms
on the International Space Station. An Insect Habitat will house Drosophila melanogaster, and in the future will accommodate other insects. It will provide an
internal centrifuge for 1 g
controls, collect video data, and allow for multigenerational studies. A general
purpose Incubator will allow for the culture of a variety of cells, tissues
and small organisms, including microorganisms and C. elegans. An Advanced Animal Habitat will house mice and rats,
and be capable of supporting multigenerational studies. A Cell Culture Unit
will accommodate microorganisms, plant and animal cells and tissues, and small
aquatic organisms. It will provide automated sampling, transfer, fixation,
and videomicroscopy capabilities. Finally, a Plant Research Unit will support
a variety of plant species, from seedlings to mature plants. All of the habitats
will provide the opportunity to manipulate the environmental conditions in
which the organisms are housed. The Plant Research Unit, the Cell Culture
Unit, the Advanced Animal Habitat and the Incubator will fit onto the large
2.5m diameter station centrifuge, providing the capability to perform 1 g controls, and intermittent gravity studies up to 2
g.
Introduction
The Space Station Biological Research Project (SSBRP) habitats will be capable of housing different species of plants, animals and microorganisms and performing long duration life sciences experiments in space (Kern, 2001). In order for these habitats to function effectively on the International Space Station (ISS), several pieces of supporting equipment will be developed. The Habitat Holding Rack will house the habitats and provide power, data and video downlinks to each habitat. The Life Science Glovebox is a critical piece of equipment that resembles a laboratory fumehood and allows the crew to perform experiments, in containment, for each habitat. The habitats either slide in underneath this glovebox, or pieces of the relevant specimen chambers are removed from the habitat in the holding rack, and then carried to the glovebox. In addition to holding the specimen chambers during experimentation, the glovebox can accomodate a microscope, a Quick-snap Freezer which allows rapid freezing of specimens, and any fluid transfer tools and containers that are specifically required by the experiment. The glovebox has a video monitor that will allow imaging from equipment such as a microscope, and facilitates experiment manipulations by the crew. The 2.5m diameter Centrifuge Rotor will allow habitats such as the Incubator, Cell Culture Unit, Advanced Animal Habitat and the Plant Research Unit to include control samples that can be maintained at artificial gravity levels of 0.01 to 2 g. The Insect Habitat contains a small diameter internal centrifuge, and therefore does not require the 2.5m diameter Centrifuge Rotor for simulating gravity loads.
An Aquatic Habitat is being developed by the Japanese Space Agency,
NASDA, and will house aquatic specimens for experimentation on the ISS. Other
SSBRP habitats that will be used to conduct life sciences experiments on the
ISS include the Biomass Production System, the Avian Development Facility
and the European Modular Cultivation System and these will be described elsewhere.
In this paper we will describe the development and capabilities of the Insect
Habitat, the Incubator, the Cell Culture Unit, the Advanced Animal Habitat
and the Plant Research Unit.
Insect Habitat
The Insect Habitat (IH) will allow scientific research with several different insect species: fruit fly, cockroach, beetle, bee, wasp, ant, butterfly, moth, cricket, and so forth. In the first few years, however, the Insect Habitat will be used for experimentation solely with Drosophila melanogaster or the fruit fly. Drosophila is a useful scientific tool for a variety of reasons. This model organism has been used by researchers for decades and therefore has sophisticated genetic tools and markers, several gene mutations that have been studied and characterized, fruit flies are fast breeding and convenient to grow, and the genome has been completely sequenced so that genes of interest can be easily identified and obtained. In addition, several processes such as nervous system function are conserved with higher organisms such as humans and rodents.
 |
| Figure 1. The Science Element (SE) consisting
of two gravity modules. (All Insect Habitat photos provided by Routes
AstroEngineering, Ottawa, Canada.) |
Within each of these gravity modules the SE is capable of holding 6 containers that will house the fruit flies. These container elements (CE) are chevron shaped and are approximately 13.5 cm (L) ´ 2.25cm (D) ´ 7.0 cm (H) in external dimensions (Figure 3). There are 3 cylinders of food located on the left, middle and right sections of the CE. The central food cylinder divides the CE into 2 chambers. The two chambers can each house two separate generations of offspring and each of the two chambers can hold up to 100 adults each. If this central food cylinder is not inserted into the CE, then the entire container can hold a total of 200 adults. These container elements can be switched in and out of the SE as subsequent generations arise.
 |
| Figure 2. A gravity module within the SE shown
in detail. A container element is shown extracted. |
The transport element or TE is the piece of hardware that will transport the container elements with the first generation of embryos, larvae or adults from Earth to ISS for experiment initiation. The TE is capable of housing up to 48 containers. The first 12 will contain the samples that will be loaded onto the SE at the start of the experiment and the remainder will contain fresh food for loading into the SE for subsequent generations. The TE will provide a controlled environment for the specimens and can be used to bring the last few generations of space-bred animals back down to Earth at the end of the increment.
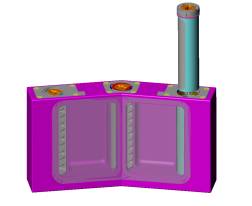 |
| Figure 3. A container ele-ment (CE) with the right
side food cylinder partially extracted. The left side and central food
cylinders are shown within the CE. |
Both the Science Element and the Transport Element will allow adjustable temperatures and relative humidity environments suitable for breeding insects. Day/night cycle lighting will be provided. Twenty-four high resolution video cameras will be available on the SE: one camera pointing at each chamber of the containers, therefore 2 cameras per container element and a total of 12 container elements in two gravity modules of the SE. These cameras will record color images during the day cycle at a resolution of 1280 ´ 1024 pixels at a rate of 30 frames per second over a field of view that covers the entire container. For capturing rapid movements of wings, appendages, and so forth, the IH video cameras will be capable of recording and storing images at 216 frames per second over an area of 128 ´ 128 pixels. Video images can also be captured during the night cycle using infrared light, which is beyond the visible range for fruit flies. More detailed descriptions of the hardware are presented in Bourdeau and Piche (2001) and in Parkes (2001).
A tool will be developed for use with the Insect Habitat that will be used to extract adults from the containers and visualize them under the dissecting microscope, or transfer to other containers to freeze the samples, or to transfer the flies to a new container for another generation of breeding.
As described above the IH contains its own internal centrifuge. This allows the sample containers to be maintained at any g level between ISS ambient and 2 g. One experimental scenario that may be used is to have half the containers maintained at microgravity, and a duplicate set of samples maintained on the other gravity module at 1g as a control. The ability to have a 1 g control in space is useful when looking at physiological effects on the animal that may be linked to radiation, vibration or other features of space travel unrelated to gravity. In addition, the flexibility of being able to command the IH from the ground mid-flight, or prior to flight with different experimental regimens, will give the researcher the option to alter the g levels at different times during flight, to look at the effects of different g levels on the organism should they choose to do so.
The Insect Habitat is capable of housing up to 200 adult fruit flies in each of its containers. There are 6 such containers per gravity module and since there are two gravity modules per science element of the Insect Habitat, the habitat is capable of flying 2400 adult flies and tens of thousands of embryos and larvae per generation in space. As the generation time is on the order of 10 days at 25oC, each three month increment will allow 7 to 9 generations of fruit flies to be bred and studied. One gets a sense then of the large number of animals that can be studied and the vast body of meaningful data that can be accumulated in each increment that the IH is used. The science that can be achieved with the IH should be both interesting and meaningful. Statistically significant numbers will be obtainable, 1g centrifuged control data will be available for every sample, multiple samples can be used for every data point.
The superb video imaging capabilities will result in large bodies of data being collected with no demands made on crew time. The resolution and frame rate of the video camera allows for visualization of fairly detailed phenotypes. This is particularly important in the context of fruit flies, where one can identify the offspring of a genetic cross by phenotypic features on the body of the animal. This will allow a lot of data to be gathered from the video records alone, with regards to behaviors and developmental status of the animals based on their genetic make-up. The rich history of research with Drosophila has resulted in the identification of a multitude of mutant lines of flies, where one gene is altered as compared to a wild type or “normal” fly. Mutants in genes expected to be affected in microgravity can be selected and used on ISS experiments. Mutant lines can be flown, they can be identified distinctly on the video images and compared to non-mutant sibling animals in the same image to determine altered behavior or development in microgravity. This will allow us to understand the roles that different gene products play in terms of adjusting to altered gravity conditions.
Fruit flies show intrinsic behaviors that are a direct response to gravity. Adults of Drosophila melanogaster move upwards in a vial on Earth, in a direction opposite to the gravity vector. Therefore one can envision seeing many behavioral anomalies when the adults are placed in microgravity since they are unable to locate the gravity vector. There are hints from past space shuttle and parabolic flight data that mobility is affected in microgravity (Miller and Keller, 1999). S. Bhattacharya’s laboratory is investigating the altered mobility resulting from hypergravity. Behavioral changes such as these can be studied on the IH with each generation of flies, through the use of the 24 video cameras and viewed by the investigator on Earth while the experiment proceeds on the ISS.
The IH will provide the opportunity to grow up to 9 generations of fruit flies on ISS. In the past, space shuttle missions have resulted in some interesting data on aging, mobility, development and behavior but due to the limitations of hardware, time, and the absence of frequent flight opportunities, some of these data are unconfirmed and some remain inconclusive. The high resolution video camera, with the ability to both store the majority of data as well as to downlink data to Earth during the increment, the capability to house large numbers of flies in the IH, and the capability to breed multiple generations will allow researchers the opportunity to thoroughly investigate the effects of microgravity.
The kinds of experiments described above can occur even in the early increments of the IH, when crew time is particularly limited and many of the additional resources of ISS still unavailable, as they require only the internal, automated video camera and occasional food changeouts by the crew.
Researchers can look at the effects of radiation on DNA. Populations of flies exposed to the space environment can be studied in order to determine the frequency and nature of the radiation effects in space. Drosophila mutants also exist that show different susceptibility to DNA damage caused by radiation as compared to wild type flies. These mutants can be tested on the ISS to determine which gene pathways are used by the organism to respond to radiation in space.
Animals can be frozen at –80oC or be fixed and brought down for subsequent analysis of the RNA on Earth. RNA is the message that is transcribed from each gene in the DNA, and is then translated into a protein molecule which performs the required function within the body. Therefore an analysis of all of the RNA made in the body at a particular point in time indicates what functions and pathways within the body will need to be active in order to react to the stresses the environment imposed on the animal at that time. This then gives scientists a list of genes and processes that are important for the body coping with a particular situation such as microgravity. The fact that the genome of Drosophila melanogaster has been sequenced in its entirety, and therefore all the genetic information is now available, will facilitate the process of analyzing gene expression patterns in microgravity.
The Insect Habitat will enable studies in the fruit fly to learn how
microgravity affects development, nervous system function and development,
movement and behavior, growth, reproduction, aging, gene expression, mutagenesis
from radiation, and circadian rhythms or sleep/wake cycles.
Incubator
The SSBRP is developing an Incubator to support a wide range
of animal, plant, cell and tissue culture and small organism experiments for
durations of up to 135 days on the ISS. The Incubator can also be used for other experiments requiring
long or short duration exposure to a temperature conditioned environment from
4 to 45°C. Prior to fabrication
of the flight units, a qualification unit will be tested with a representative
set of specimen types (Drosophila melanogaster,
Arabidopsis thatliana, Saccharomyces
cerevisiae and Caenorhabditis
elegans) to
test the biocompatibility of the design. The testing will include analysis
of growth rate, survivability and stress gene levels.
The Incubator has a usable volume of 18.8 liters, 17.2 ´ 25.9 ´
41.9 centimeters, and will have the capability to support a diverse group
of specimens from unicellular microorganisms up to small vertebrates. The
Incubator monitors the temperature and relative humidity of the specimen chamber
and can be configured to support a wide array of specimen containers (Nakamura
et al, 2001). The Incubator is being
developed by Lockheed Martin Advanced Technology Center (Palo Alto, CA) under
a contract with NASA.
One of the unique features of the Incubator is its ability
to exchange cabin air with the specimen chamber at a rate of up to 50 cc/minute.
Additionally, air is recirculated within the specimen chamber.
This feature will facilitate changes from one temperature setpoint
to another and provide a more uniform atmosphere within the chamber. Additionally,
data ports are available for five relocatable thermometers; four analog and
one digital interface; one Ethernet port and a video port; and two 60 W, 28V dc power ports. Speci-men chamber temp-erature and relative humidity sensors
can be calibrated on-orbit. Lastly,
the Incubator will func-tion in the Habitat Holding Rack (HHR), Life Sci-ences
Glovebox (LSG), and the 2.5m Centrifuge to pro-vide acceleration from microgravity
up to 2g.
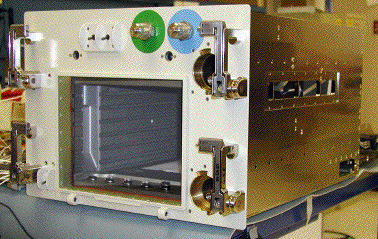 |
|
Figure 4. Incubator
|
The Incubator is designed to support experiments that will examine the effects of microgravity and space radiation on reproduction, development, graviperception and gravitropism. Additionally, the Incubator may be used to examine the relationship between temperature and fluid movements in microgravity, for analytical procedures to monitor the status of crew health and for microbial contaminant checks of the ISS. This multi-use piece of hardware will support investigations across disciplines including life sciences, human research and materials science.
The next three habitats under discussion, the Advanced Animal Habitat,
the Cell Culture Unit and the Plant Research Unit, will be developed to fly
to ISS later than the Insect Habitat and the Incubator.
Advanced Animal Habitat
The Advanced Animal Habitat-Centrifuge (AAH-C) provides an advanced research environment for laboratory rats and mice, on orbit for up to 90 days. Rodent research, previously and currently conducted by NASA during spaceflight, is limited by the capabilities of existing hardware, the ability to utilize only low Earth orbit, and short duration flights. Limited or no access to animals, and the lack of on-orbit 1-G controls have also limited the scope of rodent studies. Under development by NASA’s Space Station Biological Research Projects through a contract with STAR Enterprises, Inc. (Bloomington, IN), the AAH-C is a new rodent habitat with increased capabilities designed to address these challenges and meet the many science, animal welfare, and engineering requirements (Espinosa, et al., 2001; Kern et al., 2001).
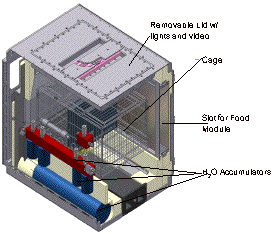 |
|
Figure 5.
The Advanced Animal Habitat schematic showing water, food, lighting
and video systems.
|
AAH-C must provide adequate delivery of food and water to the rodents, maintain adequate air flow, and control waste product odor and moisture, as well as provide investigators and crew with the ability to observe and record animal behavior. The AAH-C is internally modularized and can be reconfigured for a wide range of rodent experiments to accommodate rats and mice in all stages of their life cycle. The AAH-C will be operated in the Host Systems of the Habitat Holding Rack, on the Centrifuge Rotor, and at the Life Sciences Glovebox (for experimental procedures and manipulations). Habitat engineering data such as specimen chamber air temperature, humidity, power, food and water measurements, and light intensity will be monitored throughout the experiment. The AAH-C will provide for control of temperature, humidity, and lighting, as well as food and water delivery, and waste management for the specimens. The modular design also provides for complete change-outs of food, water, cage and waste filters. An airflow rate of at least 10 exchanges per hour prevents the accumulation of CO2 and ammonia in the specimen chamber. Air will be filtered and conditioned before exchange with the Space Station environment, to maintain bio-isolation between the crew and the specimens. Habitat parameters which are commandable from the ground include, but are not limited to, light intensity, temperature, camera on/off, air velocity, and individual animal biotelemetry sensors on/off.
Exposure to the microgravity environment of space affects a variety of organ systems in humans and other mammals. Among the most affected systems are skeletal, muscular and nervous systems. Full understanding of these effects and the testing of countermeasures (e.g., pharmacological agents, centrifugation and exercise) requires multidisciplinary, integrated research approaches in whole organisms.
AAH-C will house the most advanced laboratory specimens available for study in space to the science community. When fully developed, AAH-C will be capable of supporting rodents of all ages and life cycle stages, including pregnancy, birth, nursing, post-weaning, and adult, as well as support of multigenerational studies carried out across increments. Additionally, the habitat is designed to accommodate a variety of strains of both rats and mice, providing the opportunities for use of the most appropriate models, including transgenics, for specific research. Increased capabilities for group housing, and for flexibility in cage configuration, including tactile retreats, will allow investigators to appropriately house animals for specific experiments, limiting stress and aggressive interactions.
During ISS assembly, the AAH-C will be adaptable to support research studies of increasing complexity. The initial on-orbit experimental increment will be a verification flight, designed to provide basic data related to the functioning of the habitat and its ability to support rats in the microgravity environment. Following this initial flight, early experiments are expected to utilize 2-3 habitats, which can accommodate rats or mice on orbit. Due to crew-time limitations during the assembly phase of ISS, early experiments will require a minimal amount of crew intervention with experimental procedures. Later science experiments will be able to utilize more habitats and accommodate increasing numbers and complexity of experimental protocols. When the Centrifuge Rotor becomes available, science will be able to include centrifuge controls on a 0-2g gradient. AAH-C will provide animal biotelemetry, i.e., temperature, electrocardiograms, electromyograms, electroencephalograms, neural recordings, blood flow, blood pressure, and chemical sensor measurements. After completion of the ISS assembly, 8 AAH-C will be available on-orbit, 4 hosted on the Centrifuge and 4 in the Habitat Holding Rack. Additional ground-based units will provide earth gravity controls.
The video system will permit a level of behavioral studies never before possible in the microgravity environment. Adjustable light cycles, with infrared light for dark cycle viewing, will allow further investigation into circadian rhythms and adjustable temperatures will make it easier to accommodate a variety of strains and species. Vital long term studies with mammals are likely to focus on investigating spaceflight effects on the musculoskeletal, vestibular, cardiovascular, nervous, and immune system. While crew time limitations may preclude on-orbit dissections during early studies, the compatibility of AAH-C with the Life Sciences Glovebox will permit a variety of procedures, measurements and tissue collection to be utilized. Direct downlink of data to investigators on the ground will allow real time analysis of data and increase the interaction of principal investigators with the experiments.
The AAH-C will provide an advanced, self-contained support housing
system for rodents in the challenging environment of the ISS.
The habitat design is such that it provides for the physiological and
psychological well-being of the rodent, for general husbandry (food, water,
waste), and for the protection of the animals against the unique space environment.
It also will provide the science community with greater opportunity
and flexibility to conduct meaningful experiments in a variety of physiological
and psychological parameters affected by spaceflight and microgravity.
Cell Culture Unit
The Cell Culture Unit (CCU; Figure 6) is designed to support growth of a broad diversity of cell types including yeast, microbial and plant cells, as well as mammalian monolayer cultures (such as muscle, bone and fibroblast) and small tissue specimens. Cell biology allows the effects of spaceflight to be studied at the level of the single cell, and the CCU is a key platform for genomic and proteomics research in space biology. Yeast is just one example of a model organism whose genome is entirely sequenced and will provide an excellent opportunity to study the changes in gene expression underlying responses to spaceflight.
The CCU is designed to accommodate eighteen 3 ml, nine 10 ml, or six 30 ml cultures of animal, microbial, or plant cells, as attachment cultures or in suspension, tissue cultures (less than 4 mm in length), and non-feeding aquatic specimens. Cultures will be continuously perfused with liquid nutrient medium. Fresh, oxygenated medium will be added to each culture, and equal volumes of spent medium will be simultaneously removed. Temperature will be continuously monitored and controlled at set points between 4˚C and 39˚C, and heat shock treatment is available up to 45˚C. The pH of the media will be monitored and maintained at values between 3.5 and 8.5 pH units. Additives, including fixatives, may be introduced to the cultures. Video microscopy, selectable between 50´ and 200´, and between brightfield and differential interference contrast (DIC), will be configurable on the ground. Adherent cultures are grown on a flat glass substrate similar to a coverslip, thus facilitating the microscopic morphological analysis and recovery of the cells following experiment completion. The cell specimen containers can be removed from the CCU and taken to the Life Sciences Glovebox for manipulation including observation at 1000´ with the compound microscope. On-orbit controls will be available using the 2.5 m Centrifuge Rotor allowing one CCU to be subjected to artificial gravity of up to 2 g. Placement of the CCU on the long arm of the ISS centrifuge decreases the inertial shear stress cells experience when placed on smaller centrifuges and allows for a much more uniform g value across samples in the CCU. The CCU is the only cell culture hardware designed to work on the ISS centrifuge. Additional information can be found in de Luis et al. (2000) and Searby et al. (1998, 2001). This hardware is being built by Payloads Systems Inc. (Boston, MA) under a contract with NASA.
To take full advantage of flight opportunities, the CCU is highly automated, allowing remote manipulation, data retrieval and analysis. Automated features include the sampling module that is temperature controlled down to 4°C, self-contained video microscopy, automated subculture, media and gas exchange, additive addition, fixation of samples withdrawn from the chamber as well as washing and fixation of the cells inside the chamber. As results are analyzed on the ground, the cell environment conditions, sampling, subculture, and fixation can be reprogrammed. Remote commandability and the many automated functions decrease, and in some instances, eliminate a requirement for crew time or the presence of other equipment to complete experiments in the CCU. As a further advantage, for long term experiments and to fully utilize the 90 day window of operation on the international space station, the CCU cell chambers, sample containers and media bags can be replaced and experiments continued. These steps do require crew involvement, but can be appropriately scheduled.
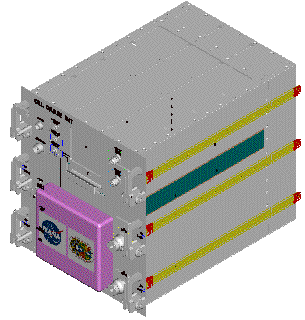 |
|
Figure 6. Design study
of the Cell Culture Unit. |
The CCU is designed to allow research into an array of cell biology questions. For example: if single cells sense changes in gravity directly, what are the intracellular structural/functional mechanisms that are sensitive to gravity perturbation? Is the cytoskeleton organization of cells disturbed by gravity perturbation? How are the following cell functions influenced by gravity and/or affected by microgravity: the expression and regulation of genetic information; cell division; cell differentiation; signal transduction, including signal-membrane interactions, membrane-effector interaction, and signal-effector linkage; membrane dynamics; intracellular transport; secretion; alternate pathway regulation; and cell-to-cell communication? These questions require microscopic visualization of cells, multiple replicate cultures and high quality of fixation of the samples taken as a result of these cultures. The CCU is designed to support this with its large individual cell chamber number (up to 18 chambers) and its pre- and post-fixation washing steps and the onboard video microscopy system that views all chambers on a rotating basis. Samples can be stored at 4°C in the thermally controlled sample module. These features should result in much greater sample quality and in the individual culture numbers necessary to accurately answer many research questions.
Because the CCU is expected to accommodate a spectrum of cell types, organisms and small tissue specimens, extensive testing with prototype hardware has been conducted. Yeast (Saccharomyces cerevisiae), BY-2 tobacco plant cells, and Euglena gracilis, a unicellular, motile algae, are suspension cell types that have been successfully grown in the CCU prototype hardware (Searby et al., 2001). Muscle organoid (muscle tissue less than 4 mm in length) is a tissue specimen that has been successfully cultivated in the prototype hardware. C2C12 murine myoblast cell line and primary human dermal fibroblasts are two examples of adherent mammalian cell types that have been cultured in the CCU hardware. The CCU is unique in the extent and type of ground testing performed during its development. The cell chambers are being tested for both adherent and suspension cultures by both the developers and by academic investigators that are independently assessing the hardware.
The CCU has been designed to accommodate a broad range of cell types
and research needs. The CCU provides an excellent opportunity
for early science because of its very low requirement for crew involvement.
CCU provides unique functions for researchers – seeding suspension cultures
on orbit, subculturing on orbit, ongoing videomicroscopy, remote commandability
and large sample numbers in combination with long duration capability.
Plant Research Unit
The Plant Research Unit (PRU) is intended to support growth of whole plants on the International Space Station for up to 90 days or longer, depending on the flight opportunities. The habitat is being built by Orbital Technologies Corporation (Orbitec) in Madison, WI. Designs for the PRU were derived from experience gained in developing the Biomass Production System (BPS). Like BPS, the PRU will contain multiple Plant Growth Chambers, each fully independent and capable of supporting growth of large whole plants.
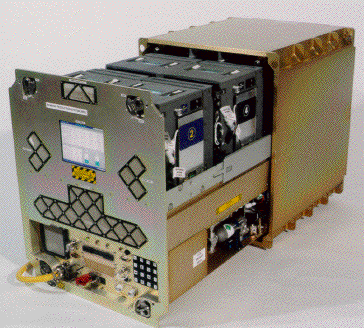 |
| Figure 7.
Biomass Pro-duction System design. The Plant Research Unit will
be modeled on this hardware. |
In its current design, the PRU will scrub ethylene from the chamber atmosphere to a level below 5 parts per billion. This low level is intended to prevent complications previously experienced in spaceflight experiments. Ethylene will be continuously removed and degraded using a photocatalytic system. Carbon dioxide control will allow for either enrichment or removal of CO2 from the chamber atmosphere.
Consistent with other habitats, the PRU will be housed in either a habitat holding rack exposed to orbital microgravity, or on the 2.5 m Centrifuge Rotor where specimens will be exposed to .01g - 2g centrifugal accelerations. Experiments can be moved between microgravity and the centrifuge, thereby providing flexibility and true gravity controls.
The PRU is designed to be self sustaining. Once the experiment is started on the ISS, automatic functionality will maintain the organisms and control environmental parameters as specified in the experimental protocol. Data can be independently acquired, stored, and reported to the ground. The habitat incorporates high resolution video and frame capture for each independent chamber. A modular design concept will allow change out of components such as LED light sources. Up to 6 habitats, each with up to 2 chambers, can fly simultaneously to provide a broad spectrum of experimental options and statistical validity.
The PRU will provide an opportunity to perform a wide array of plant
experiments on ISS. Long duration
studies of plant growth including multiple generation seed-to-seed studies
will be possible. Such prolonged
studies, performed entirely under microgravity conditions, will provide opportunity
to study the effects of gravity on fundamental plant reproductive biology
and development. Other possible
research areas include gravity sensing, signal transduction, metabolism, photosynthesis,
and transport. Growth of whole
intact plants to full maturity will provide an opportunity to study complex
topics such as induction of woodiness and mechanisms of pathogenesis.
The habitat is also capable of supporting plant tissue explants, bryophytes,
algae and other lower plant forms. Several
short duration experiments may be combined into one increment to take advantage
of research opportunities on ISS. The PRU will be adaptable to take advantage
of research opportunities provided by a suite of lab support equipment including
cryopreservation and tissue fixation. Diverse areas including classical and
molecular genetics, gene expression, anatomy, morphology, and physiology will
be supported by PRU. The habitat
will also provide a platform for research in crop production and biomass accumulation
that will be necessary for food production and waste conversion in future
long spaceflight missions.
Discussion
These life science habitats under development by the Space Station Biological Research Project will allow researchers to perform effective long duration studies in space. High resolution video cameras, multigenerational studies, perfusion based cell culture systems with efficient media flow over cells, effective air circulation to specimens, effective waste management in rodent habitats, simulated gravity (centrifuged) control samples, new sensor technology, and separate chambers with independent environmental controls are all features that will allow quality life science research aboard the International Space Station.
In plants, these studies will be valuable in order to study the effects of other stimuli such as light separately from the effect of gravity. On Earth, plants are swamped by the effect of gravity on growth, thereby making it hard to separate the effects from other stimuli. Also, plant reproduction can be studied and the quality and yield of crops and seeds can be investigated. In cell culture systems, effects have been observed in the budding patterns of yeast Saccharomyces cerevisiae (Walther et al., 1996) and also in plant, renal cortical and osteoblast cells (Staehelin et al., 2000; Hammond et al., 2000; Hughes-Fulford et al., 1998; Harris et al., 2000; Kumei et al., 1999; Sato et al., 1999) during shuttle flights. It is important to investigate these phenomena and to separate the effects of gravity from insufficient fluid flow over the cells resulting in waste accumulation and reduced nutrient concentrations in the vicinity of the cells. Rodents will continue to provide mammalian physiology data in long term space habitations and will provide clues to the cardiovascular, musculoskeletal and other phenomena experienced by the astronauts. In fruit flies, spaceflight has been reported to affect aging (Le Bourg, 1999; Benguria et al., 1996) and behavior, but hardware limitations, short duration flights, and infrequent flight opportunities have made it difficult to build a conclusive story. Hardware on the ISS that will provide for multigenerational studies, 1g controls, high resolution video cameras, numerous replicates, efficient fluid flows over cell cultures, tight environmental controls, effective air flow in specimen chambers, and so forth, will help provide consistent and interesting results.
Acknowledgments: We would like to thank Sandy Dueck, Patricia Larenas, and Chris Barreras for reading this manuscript and providing useful comments.
References: Benguria, A., Grande, E., de Juan, E.,
Ugalde, C., Miquel, J., Garesse, R., and Marco, R., 1996, J. Biotechnol. 47:
191-201; Bourdeau, J., and Piche,
L., 2001, ICES Conference Document # 01-ICES 326; De Luis, J., Vunjak-Novakovic, G., and Searby, N.D., 2000,
Proc. 51st Int. Astronaut. Congr., Rio de Janeiro, Brazil, IAF/IAA-00-G.4.06;
Espinosa, P.S., Bielitzki, J.T, Connolly, J.P., and Hinds, W.E., 2001,
ICES Conference Document 01-ICES-2228;
Hammond, T.G., Benes, E., O’Reilly, K.C., Wolf, D.A., Linnehan,
R.M., Taher, A., Kaysen, J.H., Allen, P.L., and Goodwin, T.J., 2000, Physiol.
Genomics. 3: 163-173; Harris, S.A., Zhang, M., Kidder, L.S.,
Evans, G.L., Spelsberg, T.C., and Turner, R.T., 2000, Bone, 26: 325-331; Hughes-Fulford, M., Tjandrawinata, R.,
Fitzgerald, J., Gasuad, K., and Gilbertson, V., 1998, Gravit. Space Biol.
Bull. 11: 51-60; Kern, V.D.,
Bhattacharya, S., Bowman, R.N., Donovan, F.M., Elland, C., Fahlen, T.F., Girten,
B., Kirven-Brooks, M., Lagel, K., Meeker, G.M., and Santos, O., 2001, Advances
in Space Research. 27: 1023-1030; Kumei, Y., Akiyama, H., Hirano, M., Shimokawa, H., Morita,
S., Mukai, C., and Nagaoka, S., 1999, Bio. Sci. Space. 13: 142-143; Le Bourg, E.A., 1999, Exp. Gerontol. 34:
319-336; Miller, M.S., Keller,
T.S., 1999, J. Gravit. Physiol. 6: 99-100; Nakamura, G.J., Kirven-Brooks, M., Scheller, N., 2001, ICES
Conference Document 01-ICES-329; Parkes,
T., 2001, ICES Conference Document # 01-ICES. 2232; Staehelin, L.A., Zheng, H.Q., Yoder, T.L.,
Smith, J.D., and Todd, P., 2000, Gravit. Space Biol. Bulletin. 13: 95-100;
Sato, A., Hamazaki, T., Oomura, T., Osada, H., Kakeya, M., Watanabe,
M., Nakamura, T., Nakamura, Y., Koshikawa, N., Yoshizaki, I., Aizawa, S.,
Yoda, S., Ogiso, A., Takaoki, M., Kohno, Y., and Tanaka, H., 1999, Adv. Space
Research. 24: 807-813; Searby, N.D., De Luis, J., and Vunjak-Novakovic,
G., 1998, SAE Technical Paper Series. # 981604, 28th International
Conference on Environmental Systems, Danvers, MA; Searby, N.D., Vandenriesche, J., Havens, C., Donovan, F., De
Luis, J., Pretorius, S., Lagaz, J., Sun, L., Kundakovic, L., Preda, C., Berzin,
I., and Vunjak-Novakovic, G., 2001, ICES Conference Document # 01ICES-331:
1-14; Walther, I., Bechler, B.,
Muller, O., Hunzinger, E., Cogoli, A., 1996, J. Biotechnol. 47: 113-127.
[EDITOR’S NOTE: The excellent original illustrations for this article are in color and may, therefore, not be as clear in the black and white printed version as in the color version accessible from the DIS web site: www.ou.edu/journals/dis .]