
Ashadevi, J.S., K. Ravi Ram, and S.R. Ramesh. 2001. Group-I male accessory gland secretory protein fraction is coded by autosomal gene: a study on Curly mutant of D. nasuta nasuta. Dros. Inf. Serv. 84: 91-93.
|
|
|
|||
|
|
||||
Group-I male accessory gland secretory protein fraction is coded by autosomal gene: a study on Curly mutant of D. nasuta nasuta.
Ashadevi, J.S., K. Ravi Ram, and S.R. Ramesh. Drosophila Stock Center, Department of studies in Zoology, University of Mysore, Manasagangotri, Mysore 570006, India.
Male accessory glands of insects are well known to play a key role in reproduction. in Drosophila, the secretions of accessory gland play a primary role during mating, sperm transfer and their utilization in the mated female (Fowler, 1973).
the electrophoretic patterns of these secretions are highly species-specific (Chen, 1976; Chen et al., 1985) and have been studied in different species of Drosophila such as D. melanogaster, D. simulans, D. mauritiana, D. sechellia, D. funebris, D. suzukii and in different members of the D. immigrans group (Chen, 1984; Coulthart and Singh, 1988; Chen and Balmer, 1989; Schmidt et al., 1989; Ohashi et al., 1991; Shivanna and Ramesh, 1995; Wolfner et al., 1997; Ravi Ram and Ramesh, 1999, 2001).
by SDS-PAGE analysis, Stumm-Zollinger and Chen (1985) have shown that the accessory gland secretions in D. melanogaster consist of 40 fractions, while SDS-PAGE analysis of these proteins in various members of immigrans group of Drosophila have revealed that the patterns in these species are much simpler than the pattern in D. melanogaster and they could be categorized into three groups with high molecular weight fractions falling into group-I and low molecular weight fractions into group-III (Ravi Ram and Ramesh, 1999). Further, Ravi Ram and Ramesh (2001) have reported that the secretions consist of eight major fractions in D. n. nasuta and seven major fractions in D. n. albomicans. all the studies listed here are confined only to the wild type strains. However, such an analysis has not been carried out in the mutants of any Drosophila species. When the pattern of accessory gland secretory proteins was analyzed in case of “Curly" of D. n. nasuta, an extra fraction (92 kD) was detected in addition to regular 94 kD in group-I. In the wild type of D. n. nasuta the 92 kD fraction was found to be polymorphic while only 94 kD fraction was detected in group-I of D. n. albomicans (Ravi Ram and Ramesh, 2001). Hence, the present investigations were undertaken to analyze the pattern of inheritance of 92 kD accessory gland secretory protein fraction in “Curly” of D. n. nasuta by taking advantage of differential protein patterns in group-I fractions and cross fertility between the mutant and D. n. albomicans. In the present study, we have used a wild type strain of D. n. albomicans (Okinawa, Japan, Stock No. 202.001, obtained from Drosophila Stock Center, Dept. of studies in Zoology, University of Mysore, Mysore, India) and a dominant wing mutant “Curly” (induced mutant of Mysore strain of D. n. nasuta, provided by Prof. Dr. W.-E. Kalisch, Institut für Genetik, Ruhr Universität Bochum, Bochum, Germany). these stocks were maintained at 22 ± 1ºC on standard wheat cream agar medium seeded with yeast. males and females were isolated within 4 hr of their eclosion from the pupal case. After aging them for 5 days, reciprocal crosses were conducted between “Curly” of D. n. nasuta and D. n. albomicans (wild type) to get F1 generation. The accessory gland secretory protein samples from parental as well as F1 males were prepared and SDS-PAGE patterns were analyzed following the standardized procedure (Ravi Ram and Ramesh, 2001).
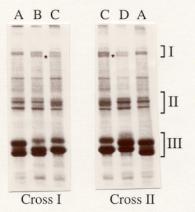 |
 |
|
Figure 1a. Group I fractions-enlarged. * = Autosomalfraction (92 kD) |
|
| Figure 1. Patterns of major accessory gland secretory
protein fractions in wild type strain of D. n. albomicans, Cy/Cy
of D. n. nasuta as well
as evidence for autosomal linkage of a major fraction in Cy/Cy
by CBB
staining; (A) D. n. albomicans,
(B) F1 male from a D. n. albomicans mother and Cy/Cy father {Cross-I}, (C) Cy/Cy,
Cross-II (D) F1 male from a D. n. albomicans father and Cy/Cy mother{Cross-II}. |
|
Acknowledgments: We
thank the Chairman of our department for the facilities. We are grateful to Prof. H.A. Ranganath
of our department and Prof. W.-E. Kalisch, Institut für Genetik, Ruhr
Universität Bochum, Germany, for stocks, encouragement, and valuable
suggestions.
References: Chen, P.S., 1976, Experientia 32: 549-550; Chen, P.S., 1984, Ann. Rev. Entomol. 29: 233-255; Chen, P.S., and J. Balmer 1989, J. Insect Physiol. 35: 759-764; Chen, P.S., E. Stumm-Zollinger, and M. Caldelari 1985, Insect Physiol. 15: 385-390; Coulthart, M.B., and R.S. Singh 1988, Biochem. Genet. 26: 153-164; Fowler, G.L., 1973, Adv. Genet. 17: 293-360; Ohashi, Y.Y., K. Hamo-Fukushima, and Y. Futuyama 1991, Insect Biochem. 21: 413-419; Schmidt, T., E. Stumm-Zollinger, P.S. Chen, P. Bohlen, and S.R. Stone 1989, J. Biol. Chem. 264: 9745-9749; Shivanna, N., and S.R. Ramesh 1995, Ind. J. Exptl. Biol. 33: 668-672; Stumm-Zollinger, E., and P.S. Chen 1985, Insect Biochem. 15: 375-383; Ravi Ram, K., and S.R. Ramesh 1999, Ind. J. Exptl. Biol. 37: 767-773; Ravi Ram, K., and S.R Ramesh 2001, Biochem. Genet. 39: 99-115; Whalen, M., and G.T. Wilson 1986, Genetics 114: 77-92; Wolfner, M.F., H.A. Harada, M.J. Bertram, T.J. Stelick, K.W. Kraus, J.M. kalb, Y.O. Lung, D. Neubaum, M. Park, and U. Tram 1997, Insect Biochem. Mol. Biol. 27: 825-834.