 |
|---|
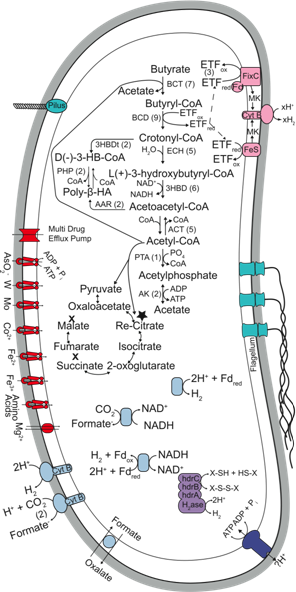
| Metabolic reconstruction of the metabolism of Syntrophomonas wolfei |
Microbial consortia are essential for the conversion of complex polymeric materials into biofuels such as methane, hydrogen, or alcohols. We are studying the physiology of microorganisms involved in biofuel production and the molecular events by which model microbial consortia respond to environmental change and regulate the flow of carbon and electrons.

Metabolic reconstruction of the
metabolism of Syntrophomonas wolfei
For more information about this program, contact the Department or Dr. Mike McInerney.
Bacterial Syntrophy
Syntrophy is an essential interaction in methane production, which involves the interaction between hydrogen- and formate-producing microbes with hydrogen- and formate-using partners. The Gibbs free energy changes involved in syntrophic metabolism are very low, close to the minimum free energy change needed to sustain microbial growth. A distinctive feature of syntrophic metabolism is the need for reverse electron transfer to produce of hydrogen or formate from electrons generated in the oxidation fatty and aromatic acids. We are using a combination of proteomic, gene expression, and biochemical approaches to (1) detect the membrane complexes involved in reverse electron transfer, (2) conduct gene expression and operon analyses to identify if key gene systems that are induced when reverse electron transfer is needed, and (3), characterize biochemically the membrane complexes involved in reverse electron transfer.
This is a collaborative effort with Professor Robert P. Gunsalus (http://www.mimg.ucla.edu/faculty/gunsalus/) and Professors Rachel and Joseph Loo at UCLA (http://www.biochemistry.ucla.edu/biochem/Faculty/Loo/). Our work will allow us to understand an essential and poorly characterized process critical for carbon cycling on the planet and will demonstrate how bacteria operate at free energy changes close to equilibrium.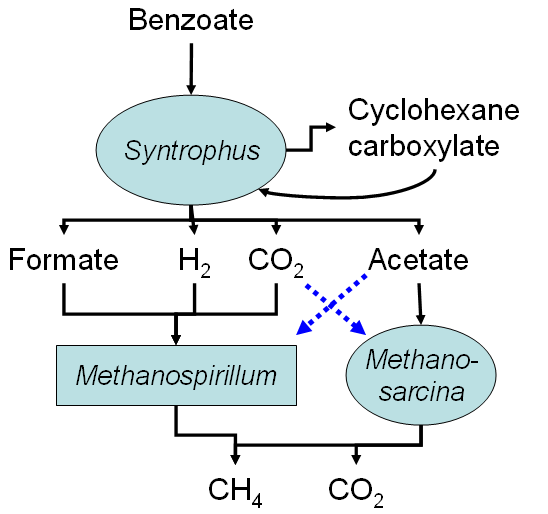
Benzoate degradation by a model syntrophic consortium. Dashed blues lines indicate additional sources of cell carbon for the methanogens
Biohydrogen Production
We are also studying biohydrogen production from renewable resources by a new microbial species called Anaerobaculum hydrogeniformans strain OS1. A. hydrogeniformans produces over 3 mol H2 per mole glucose at H2 gas phase concentrations up to 17%. We will delineate the enzymes systems involved in H2 production from carbohydrate and amino acids by A. hydrogeniformans strain OS1 and the metabolic and regulatory networks that allow efficient H2 production from glucose and amino acids. To accomplish our objectives, we are using a combination of classical biochemical approaches coupled with high-throughput technologies including genome-wide expression profiling and proteomics. Our work will provide a “systems level” understanding of the metabolic, regulatory, and physiological networks operative in hydrogen-based microbial communities. It will also allow improved assessment and predictions regarding microbially assisted, cellulosic bioconversions.
Selected Recent Publications
Other related web pages: