by Tania Vitery, Daniel Santhanaraj, Daniel Resasco, Bin Wang and Steven Crossley
Work was performed at: The University of Oklahoma and The University of Tulsa
The selective oxidation of cyclohexane to cyclohexanol and cyclohexanone was carried out using TiO2 deposited on functionalized multiwalled carbon nanotubes. Different solvents were used in continuous and biphasic systems to determine the degree of phase selectivity.
Control over selective partial oxidation is achieved through manipulation of the solvent environment surrounding the catalytic active sites.
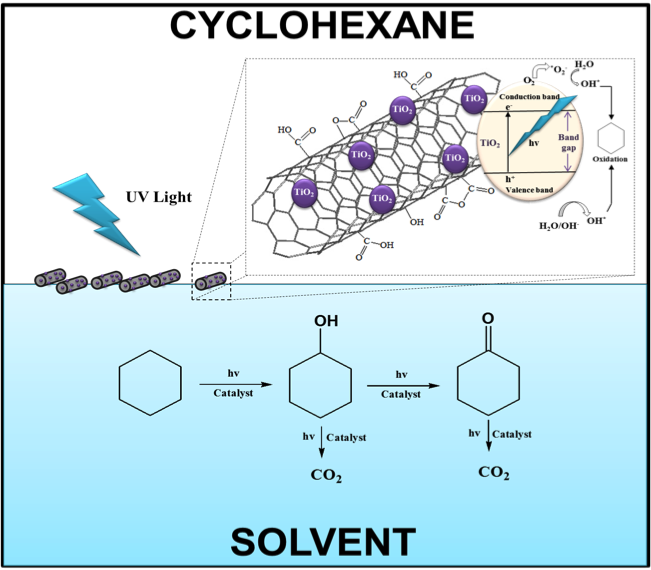
Figure: Functionalized SMW nanotubes, where TiO2 have been deposited by sol gel method, located at the oil-water interface along with the mechanisms of the activation of photocatalytic activity for cyclohexane.