 The focus of our research is on the development of different catalytic strategies for the upgrading of biofuels and production of commodity chemicals based on a new family of recoverable nanohybrid particles that catalyze reactions at the liquid-liquid interface of solid-stabilized emulsions. This approach is based on a recent discovery made by our
The focus of our research is on the development of different catalytic strategies for the upgrading of biofuels and production of commodity chemicals based on a new family of recoverable nanohybrid particles that catalyze reactions at the liquid-liquid interface of solid-stabilized emulsions. This approach is based on a recent discovery made by our
research group, in which hydrophobic carbon nanotubes fused to hydrophilic metal oxides and functionalized with catalytically active species are able to catalyze reactions in water-oil emulsions. These amphiphilic catalysts can simultaneously stabilize emulsions in biphasic liquid systems and perform catalytic reactions with high selectivity
on each phase. This unique technology is especially useful in cases in which a product might otherwise be unstable under the reaction conditions in one phase but can partition into the other phase after rapid formation. More broadly, ongoing partitioning of byproducts on the basis of their relative solubility can result in substantial simplifications at
the isolation and purification stages, obviating the need for procedures such as distillation that might damage heat sensitive compounds. Such process improvements could have a major impact in the field of biomass conversion to fuels (upgrading of pyrolysis oil and sugars), production of specialty chemicals, pharmaceuticals, deep desulfurization of fuel oil, and Fischer-Tropsch synthesis.
 This novel methodology combines heterogeneous and phase transfer catalysis, which yields to remarkable advantages, like: 1) the simplification of the refining scheme (straightforward separation of the solid catalyst and no need of surfactant), 2) the enhancement of the mass transfer of molecules between the two phases upon
This novel methodology combines heterogeneous and phase transfer catalysis, which yields to remarkable advantages, like: 1) the simplification of the refining scheme (straightforward separation of the solid catalyst and no need of surfactant), 2) the enhancement of the mass transfer of molecules between the two phases upon
emulsification, 3) the increase of the interfacial surface area, 4) and the separation of the products from the reaction mixture by differences in the water-oil solubility (preventing further decomposition).[1]
During our research several concepts have been explored, including: catalytic upgrading of bio-oil molecules and sugars, production of commodity chemicals, selective conversion of probe molecules with different solubilities, and tailoring of the catalyst and emulsion performance based on the physicochemical tuning of the nanohybrids
properties. From that work important contributions have been published in journals of great impact (Science[1], Advance Synthesis & Catalysis[2], and ChemSusChem[3]), and others are in preparation, including a patent.
In the first work, we explored two preparations with nanotubes of different type. This difference affects the deposition of Pd. As shown in Figure 1C, in the first preparation the incipient wetness impregnation method used to deposit Pd on the single-walled carbon nanotube (SWNT)–silica nanohybrids results in preferential deposition onto the silica
side. This preferential deposition is due to the very low density of defects on the Single Wall Carbon Nanotubes (SWCNT), as demonstrated by means of transmission electron microscopy (TEM) and Raman spectroscopy[1].
A more detailed characterization of the structure of these emulsions using optical and electron microscopy have been undertaken (Figure 1A-B). The SWNT-Metal Oxide nanohybrids characterized above were found to efficiently stabilize emulsions from oil/water mixtures. Figure 1(D, E, F, and G) showed the HRTEM images of the Platinum-Carbon replica obtained from the Freeze-Fracture of the emulsion stabilized by SWCNTSiO2
nanohybrid particles. The emulsions were prepared by homogenizing sonicated nanohybrids in equal volumes of water and decalin to get a concentration of 2 g/l, and further sonicating for 15 minutes. The optical microscopy image of the emulsion droplets showed that the average emulsion droplet sizes appear to be around 3-20 μm (Figure 1A).
The type of emulsion is w/o. Figure 1E presented the droplet diameter distribution of the different emulsions prepared with a water/oil ratio of 0.65/1 and 0.15 wt% SWNT-silica nanohybrids. It is expected that coalescence, sedimentation, and Ostwald ripening may lead to changes in size distribution. In fact, small changes are observed as a function of
time, evidencing the remarkable stability of this system.
The catalytic application of the Pd-containing nanohybrids to phenolic hydrodeoxygenation in a water-in-decalin emulsion was tested using vanillin (4-hydroxy- 3-methoxybenzaldehyde) as the substrate molecule, as this is a common component of pyrolysis oil derived from the lignin fraction. This compound was appealing for this
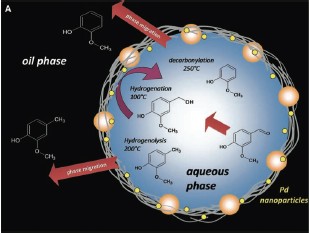
study for its different types of oxygenated functional groups (aldehyde, ether, and hydroxyl) and its partial solubility in both the organic and aqueous phases. As shown Figure 2A and 2B we observed a range of different products and phase-migration processes that were due to the varying extents of hydrogenation, hydrogenolysis, and decarbonylation reactions catalyzed by Pd as the reaction conditions were modified.
The vanillin alcohol is the primary product, but at longer times it is consumed by hydrogenolysis to form 2-methoxy-4-methylphenol (p-creosol), which migrates to the organic phase upon formation, preventing further conversion (fig. 2C).In contrast, the alcohol remains in the aqueous phase and continues reacting. This result illustrates the concept of simultaneous reaction and separation of intermediate products. Moreover the chemoselectivity changes significantly with increasing temperature, as shown in Figure 2D, at 100°C mainly hydrogenation occurs and at 200°C, hydrogenolysis becomes the dominant path even at short reaction times. At 250°C, the dominant reaction is the
decarbonylation of the aldehyde group, leading primarily to o-methoxyphenol (guaiacol).
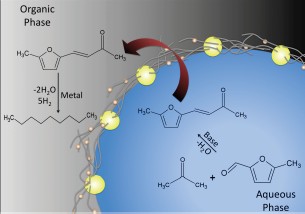
Additionally, it was explored a tandem reaction sequence in which aldol condensation of 5-methylfurfural and acetone was coupled with Pd-catalyzed hydrogenation. The former was catalyzed by MgO, which was incorporated in the
nanohybrids instead of silica. Although, the utilization of MgO, during the nanotube synthesis, generates a SWNT product that contains about 20% Multiwalled carbon nanotubes (MWNTs) as an impurity, the MgO is crucial to impart the basicity to the nanohybrids for the aldol-condensation reaction. Moreover, this nanohybrid contains nanotubes that are more defective (MWNTs) and is able to stabilize Pd particles not only on the hydrophilic side but also on the hydrophobic side. Therefore, hydrogenation can occur on both sides of the emulsion. As represented in Figure 3A, first was ran the reaction under N2 at 80°C for 3 hours and analyzed the products, which is indicated as condensation, in which no hydrogenation occurs. Self-condensation of acetone was not observed. As a result, the major product was 4-(5-methylfuran-2-yl)buten-2-one, which in line with its high log P value (~1.5) migrated almost completely to the organic phase (Figure 3B). In the second experiment, indicated as hydrogenation, an additional 1-hour reaction step at 100°C under H2 flow was added to the initial 3 hours at 80°C under N2. The 4-(5-methylfuran-2-yl)buten-2-one is totally hydrogenated in the second step, indicating that hydrogenation has occurred in the organic phase as well as in the aqueous phase. As shown in Figure 3B the carbon chains migrate to the organic phase after growing long enough to become hydrophobic, facilitating their isolation as desirable products, whereas the shorter chains remained in the aqueous phase to undergo further growth. This allow us to achieve a maximum recovery of the hydrocarbons present in the aqueous phase, that otherwise would be lost in a conventional processing scheme.
To investigate the concept of phase selectivity in emulsion systems, two aldehydeswith different solubilities were chosen, benzaldehyde (oil-soluble) and glutaraldehyde(water-soluble)[2]. The contrasting solubilities allowed us to follow the evolution of thehydrogenation activity for each molecule, simultaneously. High-resolution TEM images
were obtained to characterize the products of the two different preparations (Figure 4),and the differences are obvious. The first preparation (Figure 4A) had Pd clustersdistributed rather uniformly on the entire surface with an average particle size of 2.5 nm.The second preparation (Figure 4B) had a lower density and larger size of the of Pd
particles, deposited on one side of each silica particle (6 nm). The lower density andlarger size of Pd clusters in the second preparation can be explained by: (i) a smallerfraction of support surface available for Pd deposition, and (ii) a decreased anchoring ability of the silica support as a result of the functionalization, making sintering of themetal clusters more favorable during calcination. As shown in Figure 4C, the differencesin behavior were dramatic. When the catalyst contained Pd on both sides of the Janus particles, high conversion levels were obtained for both reactants, about 80% for glutaraldehyde in the water phase and 100% for benzaldehyde in the oil phase. However,
when the catalyst had Pd selectively deposited on the hydrophobic side, the conversion of benzaldehyde was kept at 100%, while the conversion of glutaraldehyde decreased to 2%, demonstrating high phase selectivity.
In another study, to show the enhancement in mass transfer as well as catalytic performance of the water-decalin emulsion system, we investigated the hydrogenation of p-cresol in the oil-water emulsion, single decalin and single water phase system using nanohybrid incorporated with Ruthenium. All the reactions were carried out in the same
condition, which was at 150oC, 400 psi and in one hour. The molecule p-cresol was selected because it partitions in both oil and water phases. The main product of the hydrogenation reaction is 4-methylcyclohexanol, which mostly distributes in the oil phase. The obtained reaction conversion was 61, 40 and 30% for emulsion, single water and single decalin system, respectively. The advantages of emulsion system are to diminish the aggregation of catalyst, maximize the interfacial area and improve the activity of catalyst.
These results highlight the preliminary applications of solid catalysts localized at the interface between two liquid phases. We anticipate that tailoring such emulsionstabilizing solids with additional catalytic functional groups will facilitate a broad range of reactions. Our future work will be concentrated in the understanding of the influence of the solvation effects, mass transport limitations, solvent’s competitive adsorption, and thermodynamic non-idealities in the catalytic activity and selectivity of these nanohybrid catalysts.
References:
[1] S. Crossley, J. Faria, M. Shen, D.E. Resasco, Science 1, 68 (2010).
[2] J. Faria, M. P. Ruiz, D. E. Resasco, Advanced Synthesis & Catalysis, 352, 2359
(2010).
[3] M.P. Ruiz, J. Faria, M. Shen, S. Drexler, T. Prasomsri, D.E. Resasco. ChemSusChem,
In-press (DOI: 10.1002/cssc.201000322) (2010).